Keywords
Blue crab, API, bacteria
Introduction
In some comparative studies, API system was reported to be more accurate, simple and rapid than other methods used for bacterial identification. Previously it was indicated that those system could be replaced with conventional biochemical methods (Oberhofer, 1979; Poutrel and Ryniewiczt, 1984). The API-20NE is reported to be useful for identification of environmental bacteria (Truu et al., 1999), while the API 20E system provide a reliable means to identify members of the family Enterobacteriaceae (Edwards and Ewing, 1972; Edinger et al., 1985).
It was known that the micro-organisms associated with most fishery products indicate the microbial flora in their aquatic environment (Ashie et al., 1996; Gram and Huss, 1996). It has been estimated that world ocean contain more than 1029 microbes (Whitman et al., 1998). Concentrations of microorganism of the sea depend on substantially water depth (Karl and Dore, 2001). Blue crab lives in soft-bottomed estuaries, bays and deltas, which females of the blue crab migrate offshore to spawn (FFWCC, 2005). One of the commercial important species in Akyatan lagoon on the Mediterranean coast is blue crab (Anonymous, 2008).
Fish and shellfish spoil rapidly primarily because of bacterial action (Colby et al., 1993; Sivertski et al., 2002). The shellfish flesh provides good substrate for the microorganisms due to their meat properties. Fish and shellfish may be carry pathogenic bacteria which is found in naturally aquatic environments, contaminated water or handling environments (Huss et al., 2003; Gillespie et al., 2001; Herrera et al., 2006). Thus, the purpose of this study was to investigate enteric and non-enteric bacterial flora from three different parts (crab surface, body meat and claw meat) of freshly caught blue crab using API-20E, API-20NE and API Listeria Test Kits.
Materials and Methods
Wild blue crabs (Callinectes sapidus) were caught by dip net in Akyatan Lagoon in the south of the Mediterranean Sea and transferred to laboratory. The mean carapace length and width of blue crab were 6.19±0.04 and 12.03± 0.17 cm, respectively. Blue crab samples were taken from three part of the crab which surface, body meat and claw meat. Surface of the blue crab was sampled using sterile swap and transferred onto agar plate. Body meat and claw meat of crab were aseptically weighed (10 g) and mixed with 90 mL of Ringer solution and then stomached for 3 min. Further decimal dilutions were made and then 0.1 mL of each dilution was pipetted onto the surface of agar plate in triplicate. Petri dishes used for each groups were consist of Plate Count Agar (PCA), Violet Red Bile Agar (VRBA), Listeria Sellective Agar (LSA), Salmonella-Shigella Agar (SSA). All groups were incubated for 2 days at 30ºC. Each of chosen individual bacterial colonies was spread out several times on the agar plate using sterile loop in order to produce pure colonies. Isolates were identified according to the manufacturer’s instructions of API 20E, API 20NE and API Listeria strip system (BioMereux, France). The inoculated strip was incubated for 16-24h and the colour reactions were noted either positive or negative. The result obtained were analysed using the APILAB PLUS software (Biomerieux, France).
Results and Discussion
Enteric and non-enteric bacterial flora from three different parts (crab surface, body meat and claw meat) of blue crabs were given in Table 1, 2 and 3. Total 109 bacterial strains were isolated and 20 different bacterial species were identified from fresh blue crabs. Dominant bacteria species for surface, body and claw meat of blue crab were Pseudomonas putida (25%, 6.6%, 10%), Pseudomonas luteola (12.5%, 13.3%, 10%), Pseudomonas oryzihabitans (6.25%, 6.6%, 10%), respectively. Although microflora of molluscan shellfish is more variable, the dominant bacteria were reported as pseudomonades and Moraxella/ Acinetobacter (Ashie et al., 1996; Jay, 2000, Linton et al. 2003).
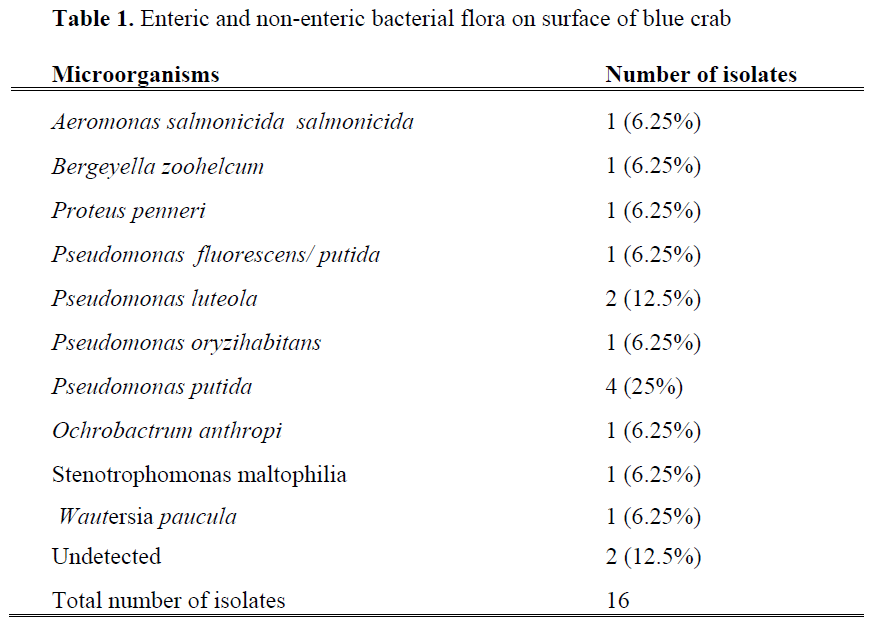
Table 1: Enteric and non-enteric bacterial flora on surface of blue crab
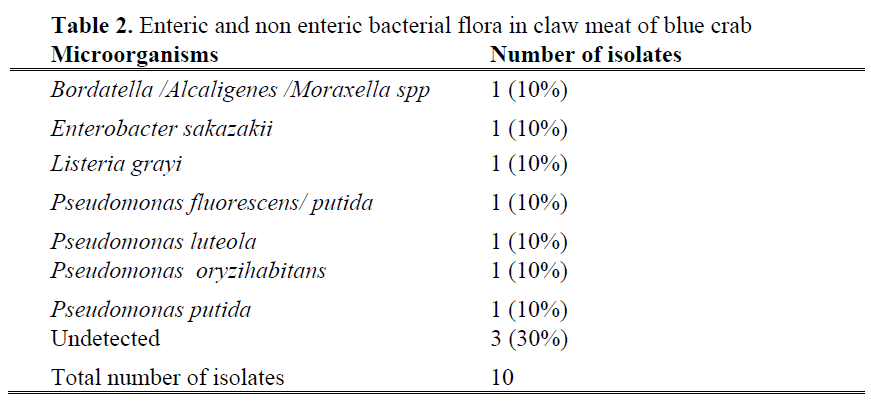
Table 2: Enteric and non enteric bacterial flora in claw meat of blue crab
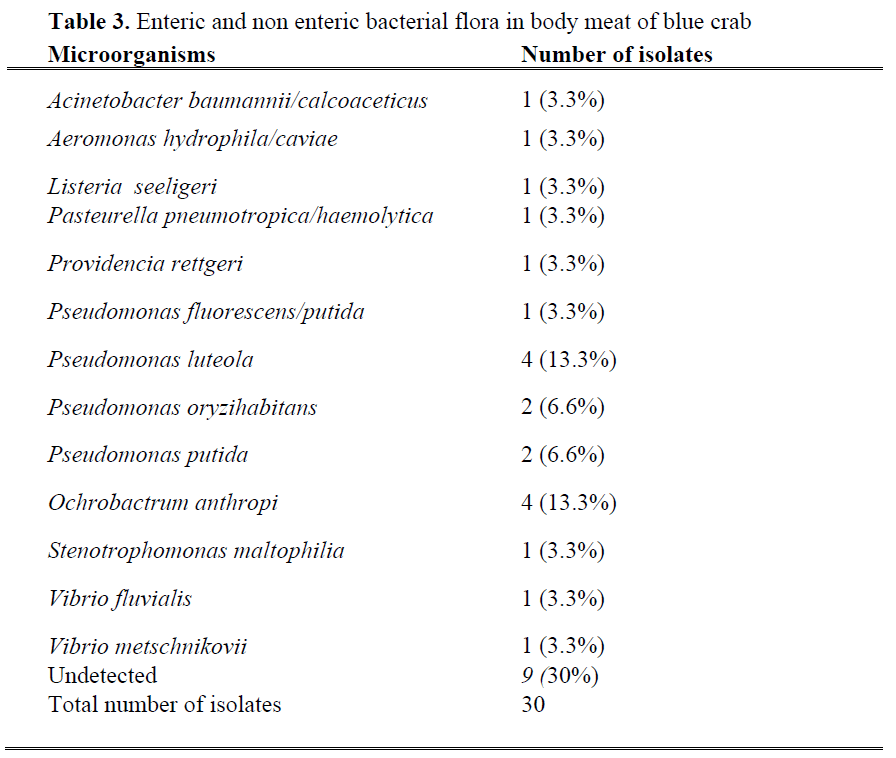
Table 3: Enteric and non enteric bacterial flora in body meat of blue crab
Bacteria of the pseudomonas genus isolated from surface of blue crab were Pseudomonas fluorescens/ putida, Pseudomonas luteola , Pseudomonas oryzihabitans and Pseudomonas putida. The remaining 37.5 % of total isolated bacteria belonged to the genera Aeromonas, Bergeyella, Proteus, Ochrobactrum, Stenotrophomonas, Wautersia. Wautersia paucula is a gram-negative environmental bacterium and causes human infection sporadically (Kwon et al., 2007), while Bergeyella zoohelcum is frequently isolated as pathogen bacteria from the upper respiratory tract of dogs, cats and other mammals (Shukla et al., 2004; Lin et al., 2007). Percentage of each of this pathogen was 6.25%. From claw meat of blue crab, Pseudomonas spp and other gram negative bacteria species such as Bordatella /Alcaligenes /Moraxella spp, Enterobacter sakazakii and Listeria grayi were isolated. Enterobacter sakazakii known as an opportunistic pathogen (FAO/WHO, 2004) recognised representing a risk to newborns, especially low birth weight and immunocompromised infants (Cordier, 2006). Listeria grayi was the only one gram positive bacteria species among the isolated strains. Jallewar et al. (2007) found 39 isolates of Listeria spp. in walking catfish (Clarias batrachus) which consisted of Listeria monocytogenes (67%), Listeria seeligeri (21%), Listeria grayi (8%) and Listeria welshimeri (5%). Listeria species isolated from fish were L. monocytogenes (1), L. innocua (6) and L. welshimeri (1) (Molla et al. 2004).
Total number of isolates from body meat of blue crab was higher than that of other parts of the blue crab. These include Acinetobacter, Aeromonas, Listeria, Pasteurella, Providencia, Pseudomonas, Ochrobactrum, Stenotrophomonas and Vibrio strains. Percentage of Pseudomonas luteola and Ochrobactrum anthropi was 13.3%, which is higher than other isolated strains. Ochrobactrum anthropi, formerly classified as Achromobacter is a ubiquitous pathogen organism, which is widely distributed in the environment and water sources (Kettaneh et al., 2003) and may be part of the normal flora of the large intestine of human (Galanakis et al., 2002; Wi et al., 2007).
Aeromonads have been isolated from various aquatic environments, including fresh and marine (saline) water systems (Krovacek and Faris, 2003). Among members of this group, Aeromonas salmonicida salmonicida and Aeromonas hydrophila/caviae constituted 6.25 % and 3.3% of total bacteria on surface and in body meat of blue crab. Although human beings are minor contributors of Aeromonas salmonicida salmonicida in the environment, aeromonads multiply to significant numbers in sewage systems (Araujo et al., 1991).
Pseudomonas, Vibrio, Aeromonas, Pasteurella, Ochrobacterium, Achromobacter, Flavobacterium, and Micrococcus were reported as dominant bacteria species in the other shellfish species such as oyster and mussel meat (Tanikawa, 1937; Colwell and Liston, 1960; Croci et al., 2001). Moreover, Serratia spp, Proteus spp. Clostridium spp. and Bacillus spp. were shown to present in crustacean meat (Jay, 2000). Bacterial flora in fresh Indian white shrimp meat was reported to be Aeromonas, Pseudomonas, Vibrio, Flavobacterium and Serratia. In that study, Aeromonas constituted about 38% of the flora (Jeyasekaran et al., 2006).
Pathogenic bacteria have been found in shellfish, including Vibrio parahaemolyticus, Salmonella, Clostridium, Escherichia coli, Campylobacter, Aeromonas and Listeria monocytogenes (Ward, 1991; Gillespie et al., 2001; Huss et al., 2000). However, in the present study, different pathogen bacteria species were isolated. The occurrence of pathogenic microorganisms in seawater or in shellfish could exist anytime sewage from human or animal origin would be discharged to the coast (Metcalf, 1982). The presence of pathogens in the environment mainly depends on the density of the coastal urban and animal populations (Pommepuy et al., 2005). The Akyatan lagoon is rich in fish and bird species. There are extensive farming activities in lagoon enviroment and receives domestic and industrial wastes. These factors could be responsible for contamination of blue crab with these different pathogen species.
Conclusions
The hygienic handing of blue crab from the moment of capture to the point of consumption is crucial to ensure good quality and long shelf life. Bacterial contamination of blue crab flesh is a main cause of spoilage and is of particular importance if the blue crab is contaminated with pathogenic bacteria, which causes food poisoning or even death amongst consumers. Therefore, blue crab should be kept clean and chill temperature which can help to minimise or delay the growth of bacteria. Good hygiene practice and proper handling are necessary to prevent food poisoning associated with consumption of blue crab.
1071
References
- Anonymous (2008). https://www.wetlands.org/reports/ris/3TR007 en_part2.pdf
- nAraujo R.M., Arribas R.M., Pares R. (1991). Distribution of Aeromonas species in waters with different levels of pollution, Journal of Applied Bacteriology, 71: 182-186. doi:10.1111/j.1365-2672.1991.tb02976.x
- nAshie, I.N.A., Smith, J.P., Simpson, B.K. (1996). Spoilage and shelf-life extension of fresh fish and shellfish, Critical Reviews in Food Science and Technology, 36: 87-121
- nColwell R. R., Liston J. (1960). Microbiology of Shellfish Bacteriological Study of the Natural Flora of Pacific Oysters (Crassostreagigas), Applied Microbiology, 8(2): 104-109
- nCroci, L. Serratore, P. Cozzi L., Stacchini A., Milandri S., Suffredini E. Ant Toti, L. (2001). Detection of Vibrionceae in mussels and in their seawater growing area, Letters in Applied Microbiology 32(1): 57-61. doi:10.1111/j.1472-765X.2001.00855.x
- nColby, J.-W., Enriquez-Ibarra, L., Flick, G.J. Jr (1993). Shelf life of fish and shellfish, in Charalambous G. (ed), Shelf Life Studies of Foods and Beverages : Chemical, Biological, Physical and Nutritional Aspects, Elsevier, 85- 143. Amsterdam
- nCordier, J.L (2006). Enterobacteriaceae. In: Emerging foodborne pathogens, eds. Motarjemi Y. and Adams M., pp.450- 475.Woodhead Publishing Limited, Cambridge, England
- nEdinger, R. C. Migneault P. C., Nolte F. S. (1985). Supplementary Rapid Biochemical Test Panel for the API 20E Bacterial Identification System, Journal of Clinical Microbiology, 22(6): 1063-1065
- nEdwards, P. R., W. H. Ewing. (1972). Identification of Enterobacteriaceae, 3rd ed. Burgess Co., Minneapolis, Minn
- nFAO/WHO (2004). Report of the meeting on Enterobactersakazakii and other microorganJournal of FisheriesSciences.com Özogul, et al., 4(1): 1-7 (2010) Journal abbreviation: J FisheriesSciences.com 6 isms in powdered infant formula, Microbiological Risk Assessment Series No. 6,Geneva(https://www.who.int/foodsafety/pu blications/micro/enterobacter_ sakazakii/en/ )
- nFFWCC ( Florida Fish and Wildlife Conservation Commission) (2005). Blue crab. Fish and Wildlife Research Institute https://research.myfwc.com/engine/download _redirection_process.asp?file=blue_crabs_4 443.pdf&objid=-3805&dltype=product
- nGalanakis E., Bitsori M., Samonis G., Christidou A., Georgiladakis A., Sbyrakis S., Tselentis Y., (2002). Ochrobactrumanthropi bacteremia in immuno competent children, Scandinavian Journal of Infectious Diseases, 34: 800-803
- nGram, L., Huss, H.H., (1996). Microbiological spoilage of fish and fish products, International Journal of Food Microbiology, 33:121-137. doi:10.1016/0168-1605(96)01134-8
- nGillespie, I.A., Adak, G.K., O’Brien, S.J., Brett, M.M., Bolton, F.J. (2001) .General outbreaks of infectious intestinal disease associated with fish and shellfish, England and Wales,. Commun Dis Public Health 4:117- 123. Herrera F.C., Santos, J.A., Otero A., Garcia- Lopez M.-L. (2006). Occurrence of foodborne pathogenic bacteria in retail prepackaged portions of marine fish in Spain, Journal of Applied Microbiology, 100: 527-536. doi:10.1111/j.1365-2672.2005.02848.x
- nHuss, H.H., Jorgensen, L.V. , Vogel, B.F. (2000). Control options for Listeria monocytogenes in seafoods, International Journal of Food Microbiology, 62:267-274. doi:10.1016/S0168-1605(00)00347-0
- nHuss, H.H., Ababouch, L. and Gram, L. (2003) Assessment and Management of Seafood Safety and Quality. Rome: FAO
- nJallewar P.K., Kalorey D.R., Kurkure N.V., Pandeb V.V. and Barbuddhe S.B. (2007). Genotypic characterization of Listeria spp. isolated from fresh water fish International Journal of Food Microbiology. 114(1): 120- 123. doi:10.1016/j.ijfoodmicro.2006.09.034
- nJay, J.M. (2000). Seafoods, in Modern Food Microbiology, 6th edn. pp.101–111, Aspen Publishers Inc.Maryland, USA
- nJeyasekaran, G., Ganesan P., Anandaraj R., Shakila R. J., Sukumar D. (2006). Quantitative and qualitative studies on the bacteriological quality of Indian white shrimp (Penaeusindicus) stored in dry ice, Food Microbiology, 23: 526-533. doi:10.1016/j.fm.2005.09.009
- nKarl, D.M., Dore JE, (2001). Microbial ecology at sea:Sampling,subsampling and incubation considerations, in Paul J.H. ed., Methods in microbiology, marine microbiology. Academic press.Aharcourt Science and Technology company. 30:13-15, London
- nKettaneh A., Weill F.-X., Poilane I., Fain O., Thomas M., Herrmann J.-L., Hocqueloux L. (2003). Septic shock caused by Ochrobactrumanthropi in an otherwise healthy host, Journal of Clinical Microbiology, 41: 1339- 1341. doi:10.1128/JCM.41.3.1339-1341.2003
- nKrovacek, K., Faris, A., (2003).Aeromonas species, in Miliotis, M.D. and Bier J.W. eds., international handbook of foodborne pathogens, 1-11. Marcel Dekker, INC, USA
- nKwon M. J., Kim J.Y., Roh K. H., Nam M. H., Yoon S.Y., Lim C.S.,. Lee C. K, Cho Y.,Kim Y.K., Lee K. N. (2007). Isolation of Wautersiapaucula from urine in a intensive care patient: A Case Report, www.kslm.org/board/lib/down.php?boardid =board_meeting&no=64-
- nLin, W. R. Chen, Y. S. Liu, Y. C. (2007). Cellulitis and bacteremia caused by Bergeyellazoohelcum, Journal Formosan Medical Association, 106(7): 573-576. doi:10.1016/S0929-6646(07)60008-4
- nLinton, M., Mc Clements, J. M. J., Patterson, M. F. (2003). Changes in the microbiological quality of shellfish, brought about by treatment with high hydrostatic pressure, International Journal of Food Science and Technology, 38: 713–727. doi:10.1046/j.1365-2621.2003.00724.x
- nMetcalf, T.G. (1982). Virus in shellfish-growing waters. Environment International, 7:21–27
- nMolla B., Yilma R., Alemayehu D. (2004). Listeria monocytogenes and other Listeria species in retail meat and milk products in Addis Ababa Ethiopia. Ethiopian Journal of Health Development, 18(3): 208-212
- nOberhofer, T. R. 1979. Comparison of the API 20E and Oxi/Ferm systems in identification of nonfermentative and oxidasepositive fermentative bacteria. Journal of Clinical Microbiology, 9:220-226
- nPommepuy M., Hervio-Heath D., Caprais M. P., Gourmelon M., Le Saux J. C., and Le Guyader F. (2005). Fecal Contamination in Coastal Areas: An Engineering Approach, in BelkinS.and Colwell R.R. Ed., Oceans and Health: Pathogens in the Marine Environment, pp. 331-359. Springer, US
- nPoutrel B., Ryniewicz H. Z. (1984). Evaluation of the API 20 Strep System for Species Identification of Streptococci Isolated from Bovine Mastitis, Journal of Clinical Microbiology, 213-214
- nShukla SK, Paustian DL, Stockwell PJ, et al. (2004). Isolation of a fastidious Bergeyella species associated with cellulitis after a cat bite and a phylogenetic comparison with Bergeyellazoohelcum strains, Journal of Clinical Microbiology, 42(1): 290-293 doi:10.1128/JCM.42.1.290-293.2004
- nSivertsvik, M. Jeksrud, W. K., Rosnes J. T. (2002). A review of modified atmosphere packaging of fish and fishery products – significance of microbial growth, activities and safety, International Journal of Food Science and Technology, 37:107-127. doi:10.1046/j.1365-2621.2002.00548.x
- nTanikawa, E. (1937). Bacteriological examination of oysters stored at low temperatures. Zentr. Bakteriol. Parasitenk. Abt. 2 (97): 133-147
- nWard, D.R., (2001). Description of the situation, Journal of Food Science, 66 (7): 1067-1071
- nWhitman, W.B., Coleman, D.C and Wiebe, W.J. (1998). Prokaryotes:The unseen majority, Proceedings of the National Academy of Sciences of the United States of America, 95: 6578-6583
- nWi, Y.M. Sohn, K-m.,Rhee, J-y., Oh, W. S., Peck, K.R., Lee, N.Y., Song, J.-H., (2007). Spontaneous bacterial peritonitis due to Ochrobactrumanthropi : A Case Report, Journal of Korean Medicine Science, 22: 377-379. doi:10.3346/jkms.2007.22.2.377









