Keywords
Basal estradiol; Follicular estradiol; Ovarian hyperstimulation syndrome (OHSS)
Introduction
Ovarian hyperstimulation syndrome (OHSS) is one of the most serious iatrogenic complications that patients undergoing controlled ovarian stimulation (EOC) treatment in assisted reproduction face. The incidence varies according to the severity of the condition, ranging from 33% of mild forms to 1% of severe cases in which the woman's life may be compromised [1].
In general it can be said that all women undergoing fertility treatment are at risk for this complication, but there are predisposing risk factors such as young age (less than 30 years), low body mass index (BMI) (<20), the presence of polycystic ovary (PCOS), elevated serum estradiol levels immediately before administration of human chorionic gonadotropin (hCG) (>3500 pg/mL) or a rapid (>50%) elevation with respect to Previous day, a high ovarian follicle count after stimulation treatment, etc. [2].
It has been observed that the basal serum anti-müleriana (AMH) level is a more reliable marker than the age and the BMI in the prediction of SHO. In addition, both the baseline serum AMH level and the serum estradiol level on the day of hCG administration are significant predictors of moderate and severe OHSS [3].
This syndrome is characterized by a persistent and prolonged increase in ovarian size due to an exaggerated response to EOC, associated with an accumulation of fluid in the third space as a consequence of an increase in capillary permeability mediated by ovarian release of the vascular factor of Endothelial growth or VEGF [4]. Although its etiology is unknown, it is well known that the triggering factor is the presence of hCG, either exogenous from its administration during hormonal treatment or from endogenous origin after gestation.
The Spanish Society of Gynecology and Obstetrics (SEGO) [5] has proposed as a quality index in assisted reproduction techniques the reduction in the incidence of severe SHO to less than 1% of stimulated cycles, hence all efforts currently Are aimed at prevention and/or early detection of this syndrome.
The objective of this study is to know the usefulness of estradiol concentrations in follicular fluid as a predictive factor of developing OHSS in women undergoing advanced assisted reproduction techniques, either IVF (conventional in vitro fertilization) or ICSI (intracytoplasmic sperm injection).
Material and Methods
A retrospective study was carried out for 6 months with 110 patients undergoing ovarian stimulation cycles in the Assisted Reproduction Unit of our hospital. Previously in accordance with professional ethics standards, couples were asked to participate in this study together with all the necessary information in the form of informed consent. In addition, the project was approved by the Hospital Ethics Committee.
The specific inclusion criteria were as follows: all women undergoing hormonal stimulation treatment in the Assisted Reproduction Unit of our hospital with subsequent transvaginal follicular puncture.
Excluded:
• Patients undergoing hormonal stimulation treatments that were not followed for follicular puncture due to clinical or facultative criteria.
• Patients undergoing second follicular punctures in our Unit (in our center usually only two ovarian punctures are performed per couple and/or woman).
• Patients whose follicular fluid was contaminated with blood.
• Patients who did not consent to participation in the project.
We performed a compilation of the following variables: age, body mass index (BMI) (weight in kg/ht in m2), smoking habit, history of polycystic ovarian syndrome (PCOS), number of mature oocytes (metaphase II) (Nausea, vomiting, respiratory distress) or signs of OOS (increased ovarian size and/or presence of intra-abdominal free fluid on abdominal ultrasound) at the time of embryo transfer. Staging of SHO severity was performed according to Golan classification (Table 1).
| SHO Light |
| Grade 1 |
Abdominal distension and discomfort |
| Grade 2 |
Similar to Grade 1 plus nausea and/or vomiting and/or diarrhea Ovaries thickened from 5 cm to 12cm |
| SHO moderate |
| Grade 3 |
Similar to mild SHO, but evidence of ascites ultrasoundSHO serious |
| SHO serious |
| Grade 4 |
Similar to moderate SHO and clinical evidence of ascites and/or hydrothorax or difficulty breathing. |
| Grade 5 |
All of the above together with hypervolemia, hemoconcentration, coagulation abnormalities and decreased perfusion and renal function |
Table 1: Golan classification ovarian hyperstimulation syndrome (1989).
In all patients, the serum estradiol concentration was determined in basal conditions (day 3 of the cycle) and in follicular fluid from a sample obtained during ovarian puncture. These determinations were performed by electro chemiluminescence on an E170 immunoanalyst from Roche Diagnostics, after calibration of the technique and meeting both the required internal and external quality controls (total precision: coefficient of variation from 2.6% to 4.7%, for estradiol concentrations of 13500 pg/ML and 32 pg/mL, respectively). This technique has a measurement range of 5 pg/mL to 4300 pg/mL, with an analytical sensitivity of 5 pg/mL and no evidence of jaundice (bilirubin <66 mg/dl), haemolysis (hemoglobin <1 g/dL), lipemia DL) or biotin (<36 ng/mL).
In the samples of follicular fluid that exceeded the linearity of the technique (>4300 pg/mL) due to their high estradiol concentrations, they were manually diluted with Elecsys Diluent Multi Assay Reagent, with an automatic pipette whose calibration had been tested in the last 6 months.
As for the hormonal treatment, short protocol of gonadotrophin releasing hormone (GnRH) antagonist was used for pituitary braking along with ovarian stimulation with FSH (225 IU/day) and HMG (2 IU/day). As ovulation inducer, hCG bolus (250 mg) was used, 36 hours prior to follicular puncture.
Statistical analysis was performed with SPSS software version 15.0. Descriptive statistics were calculated using the Kolmogorov-Smirnov test to verify the normality of variables, and Spearman's correlation to determine the association between mature oocytes and serum vs follicular estradiol. In order to study the predictive capacity of Ovarian Hyperstimulation Syndrome, serum estradiol and follicular estradiol means were compared by Mann-Whitney U-test for non-parametric discrete dichotomous variables and finally ROC curve was constructed and estradiol cutoff was calculated in follicular fluid to predict this complication.
Results
In the study, mean age of the patients was 33.9 ± 3.6 years, BMI was 25.2 ± 5.1 and 28% claimed to be habitual smokers. With regard to the analytical variables, for basal estradiol the mean was 51.3 ± 31, with 7.5 ± 6.2 mature oocytes per puncture.
In addition, as shown in the table below (Table 2) and box diagrams (Figure 1), we performed a study of the distribution of follicular estradiol levels according to age, weight and smoking groups, showing significant differences (p<0.05) in different groups.
| Variables |
Follicular Estradiol (pg/ml) |
| Age |
| ≤37 years |
874542.7±125705.7 |
| >37 years |
371191.6±18710.7 |
| BMI |
| 20-25 |
859536.2 ± 125236.2 |
| >25 |
643688.4±93588.7 |
| Tobacco |
| No |
810503.5±1250260 |
| Si |
244396.5±85995 |
Table 2: Distribution of follicular estradiol concentrations according to variables age, BMI and smoking.
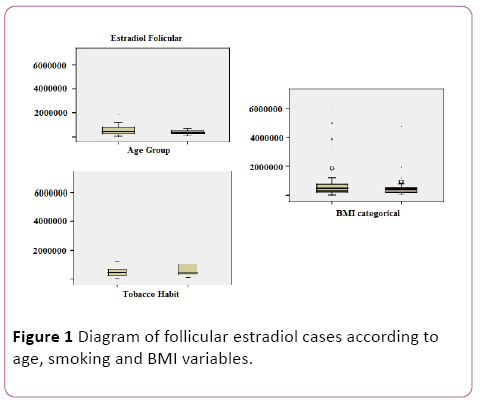
Figure 1: Diagram of follicular estradiol cases according to age, smoking and BMI variables.
Data was analyzed to determine if there was a relationship between follicular estradiol levels and the number of mature oocytes obtained after puncture and with the development of OHSS, with a Spearman correlation coefficient of 0.624 (p<0.01) and 0.893 (P<0.01) respectively, with typical errors of the estimation of 4.896 and 0.151. If, on the other hand, the correlation study was performed using baseline estradiol levels, the coefficients obtained were 0.105 (p<0.05) and 0.227 (p<0.05) respectively, with typical errors of the estimation of 6.229 and 0.327.
In the contrast of hypotheses for the comparison of serum estradiol means according to the development or nondevelopment of SHO, the obtained Mann-Whitney U (0.355) indicated that there are no significant differences in basal estradiol concentrations between the two groups. When the variable to be compared was follicular estradiol, we rejected the null hypothesis that "the mean of follicular estradiol is similar in both groups", or what is alternatively equal, "that there is a statistically significant association between the follicular concentration of estradiol And the SHO "(Mann- Whitney U of 0.001).
Next, a ROC curve for serum estradiol (Figure 2) and follicular estradiol (Figure 3) was performed, with an area under the curve of 0.583 and 0.999, respectively. For follicular estradiol the calculated cutt-off was 1515000 pg/mL, with a sensitivity of 92% and specificity of 100%.
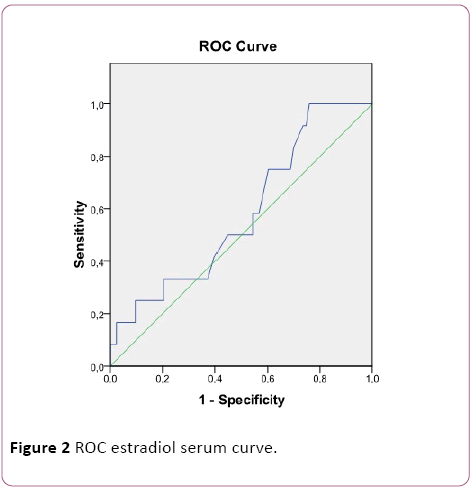
Figure 2: ROC estradiol serum curve.
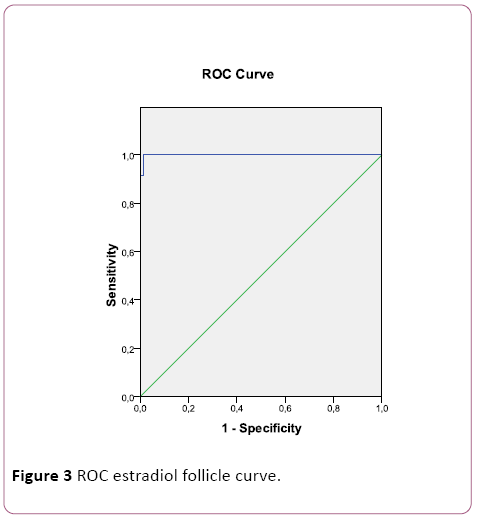
Figure 3: ROC estradiol follicle curve.
The presence of SHO was detected in 12 cases out of 110 of the study, which represents 11% of the study population. All cases were classified as mild grade (according to Golan classification), being resolved with medical treatment (rest, abundant hydration and analgesics of common use) without hospitalization. In addition, as a preventive measure, embryo Vitrification was used and embryo transfer was delayed to another non-stimulated cycle (with a biochemical pregnancy rate with cryopreserved embryos of 58%). It was observed that in 7 of the patients who developed SHO (58%) there was a previous diagnosis of PCOS, with peripheral resistance to insulin on treatment with metformin and not responding to clomiphene citrate.
Discussion
It is now well known that there are many factors influencing fertility. In this sense, the decrease of fertility with the increase of the age due to the reduction of the ovarian follicular reserve and the quality of the oocytes is totally demonstrated. Another important factor is not maintaining a normal weight (BMI between 20 and 25). Smoking also worsens reproductive prognosis, especially in women who resort to assisted reproduction techniques [6,7]. In our study, these facts become apparent when we observed significant differences between the means obtained from the follicular estradiol levels of patients according to age, smoking and BMI, reflecting a worse ovarian response to these treatments.
On the other hand, the possible relationship between the levels of serum and follicular estradiol with the number of mature oocytes collected was confirmed, confirming a positive correlation in both cases, being a weak association in the case of serum estradiol whereas with follicular estradiol the association is moderate.
In our study, short antagonist protocol was used for pituitary braking. It has been shown that, despite the fact that with a long GnRH agonist protocol, higher rates of implantation and clinical pregnancy are achieved, the incidence of OHSS is lower when GnRH antho-agonists are administered [8].
As an inducer of ovulation, bolus of hCG was used 36 hours prior to follicular puncture, which increases the risk of triggering SHO, especially in highly responding patients. For this reason, we intended to administer to group of patients with bolus of GnRH agonists instead of hCG to induce ovulation. Endogenous discharge of LH that is released after administration of the agonist bolus by its known effect "Flare up" since it has been observed that the use of GnRH agonists as an ovulation inducer reduces VEGF release, will allow us to reduce the risk of OHSS occurrence [9].
Regarding the utility of estradiol as a predictive marker of OHSS, we can state that, for both serum and follicular [10], there is a positive relationship. Thus, the higher the estradiol concentrations, the greater the risk of developing this complication. This association becomes much stronger when analyzing the levels of this hormone in follicular fluid than in basal conditions, reaching a correlation coefficient close to 0.9. On the other hand, no significant differences were observed when the measurement was performed in serum whereas they were evident when they were determined in follicular fluid of patients without SHO and with SHO.
When studying the ROC curve, it was shown the usefulness of predicting estradiol concentrations in follicular fluid with respect to basal estradiol. In addition, with a suitable cutoff point (1515000 pg/mL), a high diagnostic capacity was observed, with a sensitivity of 92% and specificity of 100%.
There are multiple articles that demonstrate the usefulness of serum estradiol concentrations on the day of induction of ovulation with hCG [11] to predict the risk of OHSS. In our case and because of work routine, it is difficult to perform the blood extraction on that day, so it was shown that we could use the sample of follicular fluid of the day of ovarian puncture as a useful specimen for the measurement of estradiol with an important predictive value of SHO.
After this study, the value of the quantification of follicular estradiol levels in patients undergoing ovarian puncture after EOC treatments is evident [12], so that we consider the implementation of its measurement to predict the risk of OHSS especially in patients with a history of PCOS and take appropriate preventive measures to prevent its occurrence.
Finally, we decided to extend this study, adding other variables such as the antral follicles count, concentrations of Antimullerian Hormone, follicle stimulating hormone values, other treatments of hormonal stimulation, etc., as factors influential or determinant for the prognosis of SHO [13].
19054
References
- Muñoz E, Portela S, Pabón D, Mollá M, Pellicer A, et al. (2008) Ovarian hyperstimulation syndrome. Pathophysiology and diagnosis. Practical Handbook on Sterility and Assisted Reproduction, III ed. Mc Graw Hill 263-270.
- Whelan JG, Vlahos NF (2000) The ovarian hyperstimulation syndrome. Fertil Steril 73: 883-896.
- Lee T, Liu C, Huang C, Wu Y, Shih Y, et al. (2008) Serum anti-muller hormone and estradiol levels as predictors of ovarian hyperstimulation syndrome in assisted reproduction technology cycles. Hum Reprod 23: 160-167
- Nastri CO, Teixeira DM, Moroni RM, Leitão VM, Martins WP (2015) Ovarian hyperstimulation syndrome: Pathophysiology, staging, prediction and prevention. Ultrasound Obstet Gynecol 45: 377-393.
- (2005) PROSEGO: Protocols and guidelines for clinical update in Gynecology and Obstetrics. Ovarian hyperstimulation syndrome. https://www. Prosego.com
- Ashrafi M,Bahmanabadi A,Akhond MR,Arabipoor A (2015) Predictive factors of early moderate/severe ovarian hyperstimulation syndrome in non-polycystic ovarian syndrome patients: A statistical model. Arch Gynecol Obstet.
- Kasum M,Oreskovi S (2011) New insights in prediction of ovarian hyperstimulation syndrome. Acta Clin 50: 281-288.
- Xing W, Lin H, Li Y, Yang D, Wang W, et al. (2015) Is the GnRH antagonist protocol effective at preventing OHSS for potentially high Responders Undergoing IVF / ICSI ?. PloS one 10.
- Casper RF (2015) Reducing the risk of OHSS by GnRH agonist triggerin. J Clin Endocrinol Metabol 100: 4396-4398.
- Broer SL, Dolleman M, van Disseldorp J, Broeze KA, Opmeer BC (2013) Prediction of an excessive response in in vitro fertilization from patient characteristics and ovarian reserve tests and comparison in subgroups: An individual patient data meta-analysis. Fertil Steril 100: 420-429.
- Siddhartha N, Reddy NS, Pandurangi M, Tamizharasi M, Radha V, et al. (2016) Correlation of serum estradiol level on the day of ovulation trigger With the reproductive outcome of intracytoplasmic sperm injection. J Hum Reprod 9: 23. 2016; 9 (1): 23-7.
- Erzincan SG, Esmer AC, Baysal B (2014) Does the estradiol level on the day of human chorionic gonadotropin administration predict the clinical outcome of controlled ovarian hyperstimulation? Clin Exp Obstet Gynecol 41: 709-712.
- Broer SL, Dolleman M, Opmeer BC, Fauser BC, et al. (2011) AMH and AFC as predictors of excessive response in controlled ovarian hyperstimulation: A meta-analysis. Hum Reprod Update 17: 46-54.









