Abbreviations
Chronic Kidney Disease (CKD)
Chronic Obstructive Pulmonary Disease (COPD)
Defined Daily Dose (DDD)
Diabetes Mellitus (DM)
Intensive Care Unit (ICU)
Methicillin Resistant Staphylococcus aureus (MRSA)
Mininum Inhibitory Concentration (MIC)
United States of America (U.S.)
Vancomycin Intermediate Staphylococcus aureus (VISA)
Vancomycin Resistant Staphylococcus aureus (VRSA)
Veterans Affairs Medical Center (VAMC)
Introduction
Vancomycin was introduced for use in the United States in 1956. Initially used for the treatment of penicillin-resistant S. aureus infections, it eventually became the drug of choice for gram positive pathogens due to the emergence of MRSA and the increase in minimum inhibitory concentrations (MICs) to nafcillin and cephalosporins [1]. Over the past decade, several authors have reported an increasing trend in vancomycin MIC levels for MRSA and methicillin-sensitive S. aureus [2-5]. The phenomenon is referred to as “vancomycin MIC creep” and has been a topic of great interest given the limited introduction of new and novel antimicrobial therapy in recent years. Other studies, however, have failed to identify the presence of MIC creep [6,7,8].
Some studies suggest that vancomycin treatment failure may be related to a higher vancomycin MIC, although the MICs are still within the susceptible range (≤2 μg/mL) [9-12]. In response, The Clinical and Laboratory Standards Institute (CLSI) in 2006 lowered the MIC susceptibility breakpoint of vancomycin from 4 mcg/mL to 2 mcg/mL, for S. aureus. Vancomycin susceptibility is currently defined as an MIC ≤2mcg/mL, vancomycin intermediate S. aureus (VISA) as an MIC equal to 4 to 8 mcg/mL, and vancomycin resistant S. aureus (VRSA) as an MIC ≥16mcg/mL [13]. Maintaining a higher vancomycin trough of >10 mg/L (15 to 20 mg/L in complicated infections such as endocarditis and bacteremia) in order to avoid treatment failure and to avoid the development of vancomycin resistance was recently recommended in a consensus guideline of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists [14].
Studies examining the effect of increased MRSA’s MIC for vancomycin on mortality and length of hospital stay have shown conflicting results [7,9,10,15,16,17,18,19]. Due to the potential for such negative outcomes from infections with MRSA with increased vancomycin MIC levels, an assessment for the presence and impact of this phenomenon is warranted. The primary goal of this study was to examine the relationship between high vs. low vancomycin MIC and patient outcomes, to assess the use of vancomycin over time, and to determine whether an ‘MIC creep’ had occurred among MRSA clinical isolates over time.
Materials and Methods
A retrospective study was conducted at the Veterans Affairs Medical Center (VAMC), a 109-bed, Midwestern, academic hospital located in Fargo, ND, from January 1, 1998 through December 31, 2008. Our facility provides health care to more than 89,000 U.S. veterans living in North Dakota, Minnesota, and South Dakota through its main campus in Fargo, ND and nine community based outpatient clinics. Services provided include general medical, surgical, orthopedic, and psychiatric care. The main facility also provides approximately fifty restorative care unit beds.
The electronic medical records of patients with blood, wound, or intravenous catheter tip cultures positive for MRSA during the study period were identified and reviewed. Variables abstracted from the electronic medical record for these patients included age, sex, site of culture, vancomycin MIC value, duration of vancomycin therapy, other antibiotics prescribed within 7 days of culture collection, and co-morbidities including diabetes mellitus (DM), chronic obstructive pulmonary disease (COPD), chronic kidney disease (CKD), malignancy, and liver disease. In the event that a patient had multiple cultures during the time period, only the last culture before the start of vancomycin of the most recent treated infection was included in the analysis.
Cultures were determined to be MRSA, by an automated Vitek machine (bioMerieux, Inc., Durham, NC). If a culture was reported as MRSA by Vitek (bioMerieux, Inc.), it was confirmed by culture on an oxacillin salt agar plate (Remel Inc., Lenexa, KS). Brain heart infusion agar (Remel Inc., Lenexa, KS) with vancomycin (6 μg/mL) was used to rule out VISA and VRSA. Vancomycin MIC values for MRSA isolates were reported by the laboratory as ≤0.5, =1, or =2 μg/mL. Catheter tip cultures were obtained through qualitative methods. Laboratory testing methods did not change over the study period.
Bacteremia was defined as at least one blood culture positive for MRSA. A wound infection was defined as at least one culture positive for MRSA. CKD was defined as a serum creatinine >2.0 mg/dL based on the lab value closest to the date of culture collection.
Vancomycin MICs were dichotomized into two groups for analysis; high MIC (≥2 μg/mL) and low MIC (<2 μg/mL). Duration of vancomycin therapy was defined as the number of days each patient received vancomycin, beginning with the first dose closest to the date of culture collection. If more than 7 days elapsed between vancomycin doses, the later dose was considered a separate treatment cycle.
Patient outcome variables included mortality and length of hospital stay. Mortality was defined as death within 30 days of the most recent MRSA culture prior to the start of vancomycin therapy. Length of hospital stay was defined as the number of days each patient remained on a hospital ward from date of admission to date of discharge. Patients were excluded from data analyses involving length of hospital stay if time was spent in the transitional care/long-term care unit for reasons other than to receive antibiotics or for treatment related to their infection. In order to assess vancomycin use over time, the Defined Daily Dose (DDD) per 100 days was calculated using a standard DDD of 2 grams. The following formula was utilized to calculate the DDD: ([Total units per year/DDD]/total patient days) x 100. SAS version 9.2 (SAS Institute Inc., Cary, NC) was used to perform statistical analyses. The dependent variable was vancomycin MIC (high or low). Bivariate comparisons were made using the t test, Chi Square test, Wilcoxon test, or Fisher exact test, where applicable. A p-value ≤ 0.05 denoted statistical significance.
Results
The inpatient population at the Fargo VAMC includes patients admitted for both elective and acute care issues including general medical, orthopedic, surgical, oncologic, and psychiatric illnesses, as well as rehabilitation services. All patients are U.S. military veterans and are predominantly male.
Two hundred and forty one MRSA cultures were identified over the study period. Ninety-seven percent (233/241) of cultures were from males. The mean patient age was 66 years (±13 years). Fifty two percent (125/241) of patients had a past medical history of diabetes, 29% (71/241) had COPD, 23% (56/241) had renal disease, and 24% (59/241) had malignancy (Table 1).
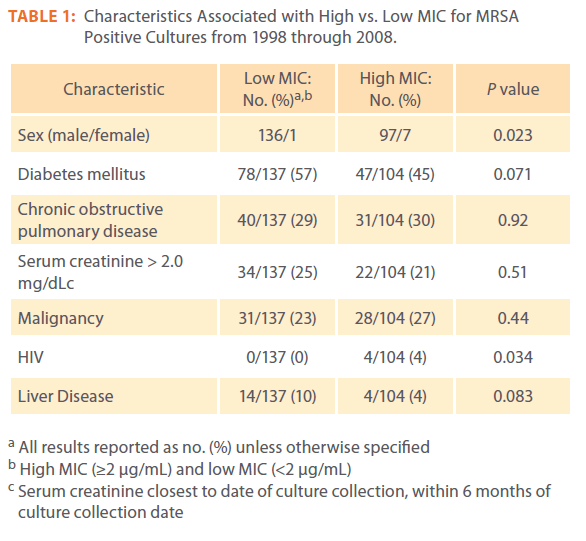
Table 1: Characteristics Associated with High vs. Low MIC for MRSA Positive Cultures from 1998 through 2008.
The creatinine clearance of two patients was not available for analysis. We were unable to determine in one patient if admission to transitional care/long-term care unit was for a reason other than infection or antibiotic administration. In regards to antibiotic use other than vancomycin, there were thirty five variables that could not be determined conclusively to have been given within one week of culture collection. Mortality within thirty days of culture was not able to be determined in one patient.
Twenty four percent (58/241) of cultures were obtained from blood and 73% (179/241) were from wound isolates and 2% (4/241) were from intravenous catheter tips. The most frequent site of wound isolates were from the lower extremity/iliac/hip (37%; 90/241) One hundred and thirty seven cultures had an MIC (<2 μg/mL). Forty one percent (56/137) of these were taken from the lower extremity/iliac/hip, 21% (29/137) were blood cultures. Nine percent (13/137) were taken from the upper extremity with 8% (11/137) coming from the Coccyx/gluteal/peri-rectal area.
One hundred and four cultures had an MIC (≥2 μg/mL). Thirtythree percent (34/104) were taken from the lower extremity/ iliac/hip, 28% (29/104) were blood cultures. Twelve percent (12/104) were from the upper extremity, with 8% (8/104) being taken from the head/neck area, and 7% (7/104) coming from the abdomen.
When concurrent antibiotic use was examined, in addition to receiving vancomycin, 39% (95/241) of patients received an antibiotic from the fluoroquinolone class within one week of culture, whereas 31% (75/241) received an antibiotic from the cephalosporin class, 30% (72/241) of patients received an antibiotic from the penicillin class, and 11% (27/241) received clindamycin. None of the antibiotics used within seven days of culture when compared with high and low MIC reached statistical significance.
We found a statistically significant upward trend in high compared to low vancomycin MICs from 1998 to 2008 (p<0.001). The numbers of low and high MIC isolates per year group were as follows: 44/6 (1998-2000), 34/24 (2001-2003), 32/37 (2004- 2006), 27/37 (2007-2008). (See Figure 1). Forty two percent (101/241) of isolates had an MIC ≤0.5, 15% (36/241) of isolates had an MIC =1, 43% (104/241) of isolates had an MIC=2.
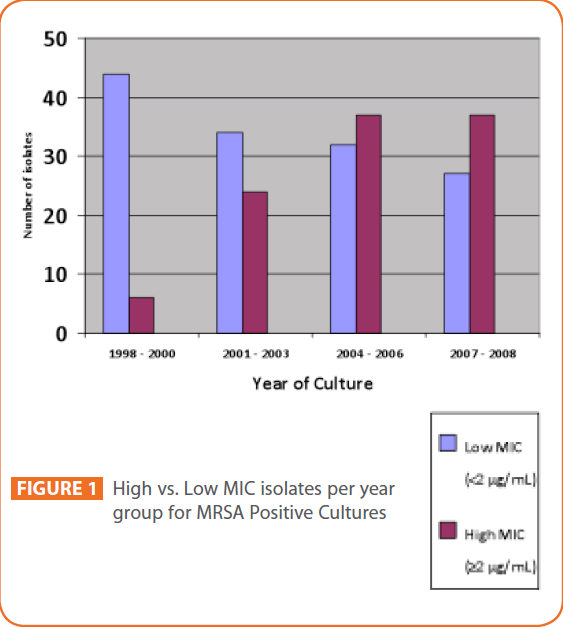
Figure 1: High vs. Low MIC isolates per year group for MRSA Positive Cultures
Past medical history of diabetes (RR=0.77; 95% CI, 0.57 to 1.02) was associated with Low vancomycin MIC compared to a high MIC, but did not reach statistical significance (p=0.07). Bivariate analysis revealed that female sex (P = 0.023) and past medical history of HIV (p=0.03) were associated with a high vancomycin MIC compared to a low MIC. History of Liver disease (RR=0.40; 95% CI= 0.17 to 0.96) was associated with low MIC compared to a high MIC but did not reach statistical significance (P= 0.083).
The median length of hospital stay was 13 days (interquartile range, 6 to 27 days). Thirteen percent (32/241) of patients died within 30 days of their most recent MRSA culture. Of these patients, all were male. Seventy one percent (27/38) of these patients had bacteremia. The median length of stay in patients who died was 21 days (interquartile range, 11 to 26 days). The mean vancomycin duration between those who died and those who survived was 8 vs. 18 days respectively (P=0.0001). Bivariate analysis did not reveal a statistically significant association between high and low MIC and 30 day mortality (p=0.21). There was no statistically significant association between length of stay and high and low MIC (p=0.5).
The DDD/100 days for each of the years from 2002 through 2009, were calculated as follows: 2.4 (2002), 2.7 (2003), 2.9 (2004), 4.0 (2005), 3.3 (2006), 4.0 (2007), 4.3 (2008), 3.7 (2009). (See Figure 2). The data required to calculate DDD was not available prior to 2002.
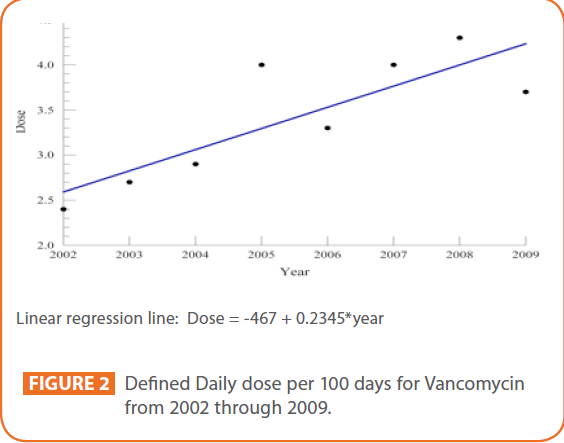
Figure 2: Defined Daily dose per 100 days for Vancomycin from 2002 through 2009.
Discussion
Staphylococcus aureus is a bacterial pathogen capable of causing multiple disease states in humans including pneumonia, sepsis, septic arthritis, toxic shock syndrome, and skin and soft tissue infections. In 2006, Steyers et al. reported that S. aureus was the most prevalent (18.7%) species isolated from inpatient specimens and the second most prevalent (14.7%) species isolated from outpatient specimens, in the United States in 2005 [20]. In a 2004 report issued by the Centers for Disease Control and Prevention, methicillin-resistant S. aureus (MRSA) isolates had increased by 11% over the previous 5 years among patients in intensive care units (ICU’s). In addition, of the nosocomial Staphylococcus aureus isolates identified in United States ICU’s in 2003, nearly 60% of these were classified as MRSA [21].
The ubiquitous nature of S. aureus combined with its ability to cause serious infections makes it an important target for decreasing morbidity and mortality. However, treatment options for MRSA are limited, with vancomycin currently being the preferred agent. Therefore the development of vancomycin resistance among MRSA is of great concern. However unequivocal data in regards to MIC creep and its association with negative outcomes is lacking.
Our reported median length of stay of 13 days with (interquartile range, 6 to 27 days) is similar to that of Lodise et al. who reported a median length of stay of 15.5 days (interquartile range 9 to 32.5 days) [10], while our finding of median duration of vancomycin therapy of 14 days (interquartile range 6 to 20 days) and an average of 17 ±16 days is similar to that of Junior et al. who reported an average duration of vancomycin therapy of 14.3 days [22].
Our finding of a statistically significant upward trend in the vancomycin MIC from 1998 to 2008 is similar to that reported in other studies. Wang et al. and Steinkraus et al. reported an increase in the vancomycin MIC value over a 5-year period. Robert et al. reported an increase in the vancomycin MIC geometric mean over a 20-year span, while Awad et al. found an increase in isolates with a MIC = 2 μg/mL over a 3-year period [2-5] .
An examination of the DDD/100 days indicates a general trend of increased vancomycin use over an 8 year period (range 2.4 to 4.3). Alos et al. also reported a similar DDD/100 days in 2002 of 1.91 [6]. However, in the Alos et al. study, vancomycin consumption remained relatively stable (range 1.64 to 2.06) from 2002 until 2006 and no MIC creep was noted. This suggests the possibility that increasing vancomycin use at our facility was a driving force for the increase in MRSA’s MIC toward vancomycin [23].
This finding of an “MIC creep” in our study however, was not associated with an increase in 30-day mortality or length of stay. Other studies have also failed to demonstrate an association between increased vancomycin MICs and negative outcome including 30 day mortality and length of stay [17,18,19]. One possible explanation for this observance was offered by Lalueza et al. who concluded in their study that MRSA isolates with a vancomycin MIC ≥1.5 were less aggressive, produced less sepsis, and less systemic inflammatory response than MRSA strains with a vancomycin MIC < 1.5 [18].
Our study also revealed that female sex (RR=2.1, 95% CI, 1.22 to 445.49) was associated with a high vancomycin MIC compared to a low MIC (P = 0.023), but due to the small number of females in the study, (n=8) generalizations to the larger population cannot be made. History of HIV was also associated with high vancomycin MIC compared to a low MIC (P = 0.034), but again due to the small number of patients with this disorder (n=4), generalizations cannot be made. History of Liver disease (RR=0.40; 95% CI, 0.17 to 0.96) was associated with low compared to a high MIC but did not reach statistical significance (P = 0.083) and its application to the general population is limited by the small numbers (n=14).
There was a significant difference in mean vancomycin duration between those who died and those who survived (P=0.0001), 8 vs. 18 days respectively. It is possible that the patients who died within 30 days of culture collection did so before the full course of vancomycin therapy could be completed, or the prescribed duration of vancomycin therapy was inadequate to treat the infection.
Limitations of this study include its retrospective nature making it difficult to control for confounding factors and the small numbers of some variables. In addition, the almost exclusive male population limits the generalizability of these findings. As a result, further studies are warranted. We recommend a larger randomized prospective study from a general hospital, as well as studies examining vancomycin MIC creep with prescribing habits.
In conclusion, a significant upward trend in vancomycin MIC values over an 11-year period was identified at our institution. This trend was not associated with increased 30-day mortality or increased length of stay. Over the last eight years of the study period, the relative consumption of vancomycin (DDD/100 days) steadily increased (range 2.4 to 4.3) providing a possible driving force for the increase in MIC. Larger randomized prospective studies would be helpful to substantiate these findings.
Acknowledgements
We wish to acknowledge Dr. Marilyn Klug from the Center for Rural Health at the University of North Dakota School of Medicine and Health Sciences for her assistance with the linear regression analysis.
Funding
No outside funding was provided for this study.
Competing Interests
No competing interests exist.
218
References
- Stevens DL (2006) The role of vancomycin in the treatment paradigm. Clin Infect Dis 42: S51-S57.
- Awad SS, Elhabash SI, Lee L, Farrow B, Berger DH (2007) Increasing incidence of methicillin-resistant Staphylococcus aureusskin and soft-tissue infections: reconsideration of empiric antimicrobial therapy. Am J Surg 194: 606-610.
- Robert J, Bismuth R, Jarlier V (2006) Decreased susceptibility to glycopeptides in methicillin-resistant Staphylococcus aureus: a 20 year study in a large French teaching hospital, 1983-2002. J AntimicrobChemother 57: 506-510.
- Steinkraus G, White R, Friedrich L(2007) Vancomycin MIC creep in non-vancomycin intermediate Staphylococcus aureus(VISA), vancomycin-susceptible clinical methicillin resistant S. aureus(MRSA) blood isolates from 2001-05. J AntimicrobChemother 60: 788-794.
- Wang G, Hindler JF, Ward KW, Bruckner DA (2006) Increased vancomycin MICs for Staphylococcus aureusclinical isolates from a university hospital during a 5-year period. J ClinMicrobiol 44: 3883-3886.
- Alós J, García-Cañas A, García-Hierro P, Rodríguez-Salvanés F (2008) Vancomycin MICs did not creep in Staphylococcus aureusisolates from 2002 to 2006 in a setting with low vancomycin usage. J AntimicrobChemother 62: 773-775.
- Musta AC, Riederer K, Shemes S, Chase P, Jose J, Johnson, LB, Khatib R (2009) Vancomycin MIC plus heteroresistance and the outcome of methicillin-resistant Staphylococcus aureusbacteremia: trends over 11 years. J ClinMicrobiol 47: 1640-1644.
- Holmes R, Jorgensen J, (2008) Inhibitory Activities of 11 Antimicrobial Agents and Bactericidal Activities of Vancomycin and Daptomycin against Invasive Methicillin-Resistant Staphylococcus aureusIsolates Obtained from 1999 through 2006, Antimicrobial Agents and Chemotherapy 52: 757–760.
- Hidayat LK, Hsu DI, Quist R, Shriner KA, Wong-Beringer A (2006) High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureusinfections. Arch Intern Med 166: 2138- 2144.
- Lodise TP, Graves J, Evans A, Graffunder E, Helmecke M, Lomaestro BM, Stellrecht K (2008) Relationship between vancomycin MIC and failure among patients with methicillin-resistant Staphylococcus aureusbacteremia treated with vancomycin. Antimicrob Agents Chemother 52: 3315-3320.
- Neoh H, Hori S, Komatsu M, Oguri T, Takeuchi F, Cui L, Hiramatsu K (2007) Impact of reduced vancomycin susceptibility on the therapeutic outcome of MRSA bloodstream infections. Ann ClinMicrobiolAntimicrob. Available: https://www.ncbi.nlm.nih.gov/ pmc/articles/PMC2148052/?tool=pubmed. Accessed 21 October 2009.
- Sakoulas G, Moise-Broder PA, Schentag J, Forrest A, Moellering RC Jr, Eliopoulos GM (2004) Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureusbacteremia. J ClinMicrobiol 42: 2398-2402.
- Tenover FC, Moellering RC Jr (2007) The rationale for revising the clinical and laboratory standards institute vancomycin minimal inhibitory concentration interpretive criteria for Staphylococcus aureus. Clin Infect Dis 44: 1208-15.
- Rybak M, Lomaestro B, Rotschafer JC, Moellering R Jr., Craig W, Billeter M, Dalovisio JR, Levine DP (2009) Therapeutic monitoring of vancomycin in adult patients: A consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 66: 82-98.
- Maclayton DO, Suda KJ, Coval KA, York CB, Garey KW (2006) Case-control study of the relationship between MRSA bacteremia with a vancomycin MIC of 2 μg/mL and risk factors, costs, and outcomes in inpatients undergoing hemodialysis. ClinTher 28: 1208-1216.
- Soriano A, Marco F, Martínez JA, Pisos E, Almela M, Dimova VP, Alamo D, Ortega M, Lopez J, Mensa J (2008) Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureusbacteremia. Clin Infect Dis 46: 193-200.
- Peleg AY, Monga D, Pillai S, et al. (2009) Reduced susceptibility to vancomycin influences pathogenicity in Staphylococcus aureus infection. JID 199: 532-6.
- Lalueza A, Chaves F, San Juan R, DaskalakMi, Otero J, Aguado J (2010) Is High Vancomycin Minimum Inhibitory Concentration a Good Marker to Predict the Outcome of Methicillin?Resistant Staphylococcus aureusBacteremia? The Journal of Infectious Diseases 201: 311-312.
- Price J, Atkinson S, Llewelyn M, Paul J (2009) Paradoxical relationship between the clinical outcome of Staphylococcus aureus bacteremia and the minimum inhibitory concentration of vancomycin. Clinical Infectious Diseases 48: 997-8.
- Styers D, Sheehan D, Hogan P, Sahm D (2006) Laboratory-based surveillance of current antimicrobial resistance patterns and trends among Staphylococcus aureus: 2005 status in the United States. Annals of Clinical Microbiology and Antimicrobials. Available: https://www.ann-clinmicrob.com/content/5/1/2. Accessed 1 June 2010.
- Centers for Disease Control and Prevention National Nosocomial Infections Surveillance System Report, data summary from January 1992 – June 2004, issued in October 2004. Am J Infect Control 32: 470-485.
- Junior M, Correa L, Marra A, Camargo L, Pereira C (2007) Analysis of Vancomycin use and Associated Risk Factors in a University Teaching Hospital: A Prospective Cohort Study. BMC Infectious Diseases. Available: https://www.biomedcentral.com/1471- 2334/7/88
- Borg MA, Zarb P, Scicluna EA, Rasslan O, Gür D, Ben Redjeb S, Elnasser Z, Daoud Z (2010) Antibiotic consumption as a driver for resistance in Staphylococcus aureus and Escherichia coli within a developing region. Am J Infect Control 38: 212-6.








