Abdul Waheed Khan1*, Fazli Zahir1, Zeeshan Nasim1,2, Muhammad Adil Khan3, Muhammad Abdullah4 and Abdul Haleem Khan5
1Institute of Biotechnology and Genetic Engineering, The University of Agriculture, Peshawar, Pakistan
2College of Life Sciences and Biotechnology, Korea University, Seoul, Korea
3Australian Research Council, Center of Excellence in Plant Energy Biology, The University of Western Australia, Perth, Australia
4Department of Zoology, Hazara University, Mansehra, Khyber Pakhtunkhwa, Pakistan
5Department of Zoology, Islamia College Peshawar, Pakistan
*Corresponding Author:
Abdul Waheed Khan
Institute of Biotechnology and Genetic Engineering
The University of Agriculture, Peshawar, Pakistan
Tel: +92-3349164181
E-mail: khan_ibge@yahoo.com
Received date: November 25, 2015 Accepted date: December 21, 2015 Published date: December 30, 2015
Copyright: © 2015, Khan AW, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Keywords
Hepatitis C; Coinfection; Vertical transmission; Pediatrics
Introduction
Hepatitis C is a major public health concern, caused by Hepatitis C Virus (HCV). Globally, the number of anti-HCV positive people is estimated to be greater than 185 million [1], with about 350,000 annual death toll due to liver associated complications [2]. Pakistan carries the burden of about 10 million HCV infected people [3].
In pediatrics, the prevalence of HCV infection varies from 0.05% to 0.36% in developed countries and between 1.8% and 5% in developing world [4]. Vertical transmission is a leading cause of childhood HCV infection [5]. During pregnancy, HCV transmission frequency from chronically infected mothers to the infant is about 5% [6]. Coinfection with Human Immune Deficiency Virus (HIV) is known to enhance the risk of vertical transmission of HCV [7]. Here, we present a case of HCV vertical transmission in HCV/HIV coinfected patient with HCV subtype 1a.
Case Presentation
In February 2013, a 32 years old lady (BMI value 21.4 kg/m2) belonging to Khwazakhela region of district Swat vaginally delivered a male infant at Saidu Teaching Hospital, Swat. Prior to delivery, the lady was declared HCV/HIV coinfected in her first trimester screening reports and administered with anti- HIV therapy only. She was discharged few hours after initial checkup of the infant (birth weight: 2.9 kg; first minute heart rate: 93 beats/min; body color: pink). In her next visit after four weeks of the delivery, a detailed examination of the infant was carried out and was found positive for anti-HCV. The Liver Function Tests (LFTs) such as Alanine Amino Transferase (ALT) and Aspartate Amino Transferase (AST) of the infant were also determined (ALT: 36 U/L; AST: 40 U/L; children normal range [8]: ALT: 10-55 U/L; AST: 10-40 U/L). For assessment of active infection, Polymerase Chain Reaction (PCR) test was carried out but the result indicated absence of viral RNA in blood of the infant. Earlier during pregnancy, the lady was identified with HCV subtype 1a. During her last PCR report the lady retained high viral titer of HCV and HIV (Table 1). Upon further inquiry, she described that previously (about four years ago) she had completed treatment regimen for HCV infection (IFN- α plus local herbal formulation for 24 weeks). Interestingly, the treatment history of the lady indicated the achievement of Sustained Virological Response (SVR) for HCV subtype 3a. At this stage, written consent was taken from the infected mother and her husband for pursual of this study.
By the end of 2nd month, the infant was subjected to various laboratory investigations and the PCR report identified active infection for the first time. The genotyping assay indicated the same HCV subtype as that of the mother i.e subtype 1a. In the following interview session, the lady revealed of carrying out three dental surgeries during the last two years. By the sixth month and onward, the infant viremia was constantly detected positive for HCV. The infant follow up session consisted of 22 months (Figure 1) from the time of birth.
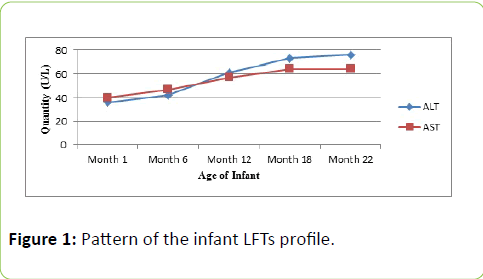
Figure 1: Pattern of the infant LFTs profile.
Discussion
To the best of our knowledge, this is the first report related to vertical transmission of HCV subtype 1a in HCV/HIV coinfected patient from Pakistan. Different studies have shown the transmission of HCV from carrier mother to infant in various regions [9-11]. As the precise timing and phenomena of HCV vertical transmission remains unclear [5] it is believed that the transmission event occurs during late intrauterine time or at birth [5,12]. Certain factors are thought to intensify the probability of HCV vertical transmission. In most vertically transmitted HCV cases, it has been observed that the infected mother used to be coinfected with HCV/HIV [13,14] compared to those infected with HCV alone [9]. According to metaanalysis of multiple studies, it is clear that HCV/HIV coinfection during pregnancy period enhances the chance of vertical transmission by 90% [13]. Previously, it has been reported that the HIV status of expecting women correlate with HCV viremia which contributes to mother to infant transmission [10]. The presence of high maternal HCV viremia observed in the current study (Table 1) is also an important factor which may aid the HCV vertical transmission [15].
| Investigation |
Gestation age |
| Month 3 |
Month 5 |
Month 8 |
| Anti-HCV |
+ |
+ |
+ |
| HCV viral titer (IU/mL) |
1432,000 |
1586,000 |
1666,000 |
| CD4+ count (cells/µL) |
311 |
332 |
348 |
| HIV titer (log10 copies/mL) |
3.82 |
3.64 |
3.49 |
Table 1: Laboratory profile of HCV/HIV coinfected mother.
In this study, HCV subtype 1a was found to be transmitted vertically, relatively an uncommon subtype in Pakistan, comprising 1.5% of the distributed HCV subtypes [16]. Some studies have highlighted frequent vertical transmission of this subtype [9], however, there is no obvious evidence of considerable HCV vertical transmission risk association with a particular HCV genotype [5,10].
Mode of delivery at the time of birth is also considered vital in the transmission of HCV from infected mother to the upcoming infant. Generally, infants delivered vaginally by HCV infected mothers have high risk of HCV acquistion [15]. Gibb et al. [17] reported 7.7% HCV vertical transmission cases in vaginally delivered infants compared to 0% in women who underwent elective cesarean section delivery while 5.9% in those who preferred emergency cesarean section.
The incubation phase in acute HCV infection varies from 30 to 60 days [7]. In the current case, anti-HCV antibodies were first detected in the infant blood by the 4th week of birth. On the contrary, serological investigation of HCV is not plausible during infancy period due to persistance of passively transmitted maternal antibodies up to 18 months [18], however, presence dof detectable viremia (Figure 2) confirmed active infection. A systemic review of 77 cohort studies of HCV infected pregnant women revealed net vertical transmission rate of 1.7% in anti-HCV positive women and 4.3% for HCV viremic women [19]. Moreover, spontaneous clearance of HCV occurs in approximately 25% infected infants while the remaining 75% develop infection of mild nature with few numbers progressing toward related liver complications [6].
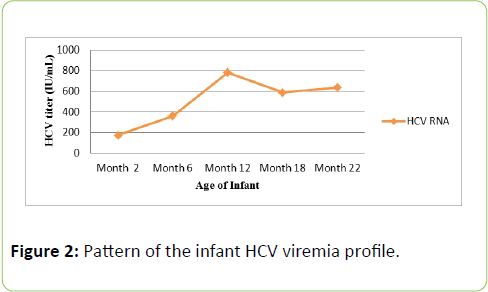
Figure 2: Pattern of the infant HCV viremia profile.
One aspect of the current study shows the lack of crossgenotype immunity for HCV subtypes in the reinfected mother (subtype 3a followed by subtype 1a). HCV genotypes are considered important in determining anti-HCV treatment duration and response to treatment regimen [20]. It has been identified that individuals retaining HCV genotype 1 are comparatively more resilient to interferon therapy than individuals infected with genotype 2 or 3 [20]. Similarly, it is suggested that the mode of acquisition in subtype 3a and 1a is associated with intravenous drug use while subtype 1b is mostly acquired via blood transfusion [21]. However, infection with one HCV genotype does not confer protection against reinfection with the same subtype or other genotypes [22,23].
In HCV/HIV coinfected prospective women, due to severe contraindications of the currently available HCV therapies during pregnancy, no anti-HCV therapy is recommended for child-bearing women [24]. In fact, interferon may cause postpartum depression in pregnant women as depression is a known side effect associated with interferon treatment [25]. Similarly, the other essential component of all anti-HCV combinations (i.e. both including and excluding interferon) is ribavirin, but having pregnancy class X status (teratogenic effect in many animals model) [26], it is also not prescribed to pregnant women. In many situations, the anti-HCV therapy is given before conception to achieve an SVR, and therefore, decline the probability of vertical transmission [27]. Nevertheless, SVR is not achieved by all patients, and thus the risk of transmission exists in viremic women [27]. Although, highly active antiretroviral therapy is useful in decline HIV titer and eventually transmission to neonate, it is not clear whether risk of HCV vertical transmission is also reduced significantly [28].
Despite the important status of hepatitis C in human health, the lack of vaccine against HCV till date and the contraindication of the currently available treatment regimens in pregnancy pose a great risk in prospect of vertical transmission. It is assumed that in the upcoming few years the currently available treatment regiments will be replaced by interferon and ribavirin free combinations which will be equally beneficial in treating pregnant women to reduce the risk of HCV vertical transmission efficiently.
Acknowledgements
The authors highly acknowledge the support of Dr. Muhammad Fahim in drafting the article.
Authors contribution
Study concept and design: AWK. Analysis and interpretation of data: AWK, ZN and FZ. Manuscript compilation: AWK, FZ, MAK, MA and AHK. Critical revision of the manuscript: MAK and MA. Technical support: AHK.
7866
References
- MohdHanafiah K, Groeger J, Flaxman AD, Wiersma ST (2013) Global epidemiology of hepatitis C virus infection: New estimates of age?specific antibody to HCV seroprevalence. Hepatology 57: 1333-1342
- Perz JF, Armstrong GL, Farington LA, Hutin YJF, Bell BP (2006) The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol 45: 529-538
- Anjum S, Ali S, Ahmad T, Afzal MS, Waheed Y, et al. (2013) Sequence and structural analysis of 3'untranslated region of hepatitis C virus, genotype 3a, from pakistani isolates. Hepat Mon 13: e8390
- Yeung CY, Lee HC, Chan WT, Jiang CB, Chang SW, et al. (2014) Vertical transmission of hepatitis C virus: Current knowledge and perspectives. World J Hepatol 6: 643
- Indolfi G, Resti M (2009) Perinatal transmission of hepatitis C virus infection. J Med Virol 81: 836-843
- Robinson J (2008) Vertical transmission of the hepatitis C virus: current knowledge and issues. Paediatr Child Health 13: 529-534
- Airoldi J, Berghella V (2006) Hepatitis C and pregnancy. ObstetGynecolSurv 61: 666-672
- Thapa BR, Walia A (2007) Liver function tests and their interpretation. Indian J Pediatr 74: 663-671
- Mast EE, Hwang LY, Seto DS, Nolte FS, Nainan OV, et al. (2005) Risk factors for perinatal transmission of hepatitis C virus (HCV) and the natural history of HCV infection acquired in infancy. J Infect Dis 192: 1880-1889
- Zanetti A, Paccagnini S, Principi N, Pizzocolo G, Caccamo M, et al. (1995) Mother-to-infant transmission of hepatitis C virus. Lancet 345: 289-291
- Mariné-Barjoan E, Berrébi A, Giordanengo V, Favre SF, Haas H, et al. (2007) HCV/HIV co-infection, HCV viral load and mode of delivery: risk factors for mother-to-child transmission of hepatitis C virus? AIDS 21: 1811-1815
- Mok J, Pembrey L, Tovo PA, Newell ML (2005) When does mother to child transmission of hepatitis C virus occurs? Arch Dis Child Fetal Neonatal Ed 90: F156-F160
- Polis CB, Shah SN, Johnson KE, Gupta A (2007) Impact of maternal HIV coinfection on the vertical transmission of hepatitis C virus: a meta-analysis. Clin Infect Dis 44: 1123-1131
- Cottrell EB, Chou R, Wasson N, Rahman B, Guise JM (2013) Reducing risk for mother-to-infant transmission of hepatitis C virus: a systematic review for the US Preventive Services Task Force. Ann Intern Med 158: 109-113
- Okamoto M, Nagata I, Murakami J, Kaji S, Iitsuka T, et al. (2000) Prospective reevaluation of risk factors in mother-to-child transmission of hepatitis C virus: high virus load, vaginal delivery, and negative anti-NS4 antibody. J Infect Dis 182: 1511-1514
- Ali S, Ali I, Azam S, Ahmad B (2011) Frequency distribution of HCV genotypes among chronic hepatitis C patients of Khyber Pakhtunkhwa. Virol J 8: 193
- Gibb DM, Goodall RL, Dunn DT, Healy M, Neave P, et al. (2000) Mother-to-child transmission of hepatitis C virus: evidence for preventable peripartum transmission. Lancet 356: 904-907
- Yeung L, To T, King S, Roberts E (2007) Spontaneous clearance of childhood hepatitis C virus infection. J Viral Hepat 14:797-805
- Robert EA, Yeung L (2002) Maternal-infant transmission of hepatitis C virus infection. Hepatology 36: S106-S113
- Zein NN (2000) Clinical significance of hepatitis C virus genotypes. ClinMicrobiol Rev 13: 223-235
- Pawlotsky JM, Tsakiris L, Roudot-Thoraval F, Pellet C,Stuyver L, et al. (1995) Relationship between hepatitits C virus genotypes and sources of infection in patients with chronic hepatitis C. J infect Dis 171: 1607-1610
- Harcourt G, Lucas M, Godkin AJ, Kantzanou M, Phillips R, et al. (2003) Evidence for lack of cross?genotype protection of CD4+ T cell responses during chronic hepatitis C virus infection. ClinExpImmunol 131: 122-129
- Aitkin CK, Lewis J, Tracy SL, Spelman T, Bowden DS, et al. (2008) High incidence of hepatitis C virus reinfection in cohort of injection drug users. Hepatology 48: 1746-1752
- Valladares G, Chacaltana A, Sjogren M (2010) The management of HCV-infected pregnant women. Ann Hepatol 9: 92-97
- Arshad M, El-Kamary SS, Jhaveri R (2011) Hepatitis C infection during pregnancy and the newborn period-are they opportunities for treatment? J Viral Hepat 18: 229-226
- Prasad MR, Honegger JR (2013) Hepatitis C virus in pregnancy. Am J Perinatol 30: 149-159
- Lange CM, Zeuzam S (2013) Perspectives and challenges of interferon-free therapy for chronic hepatitis C. J Hepatol 58: 583-592
- European Paediatric Hepatitis C virus Network (2005) A significant sex-but not elective cesarean section-effect on mother-to-child transmission of hepatitis C virus infection. J Infect Dis 192: 1872-1879







