Keywords
Vitamin B12 deficiency; Massive pulmonary embolism; Hyperhomocysteinemia; Posterior column of spinal cord; Radiology of vitamin B12 deficiency.
Introduction
The terminal ilium is the site for vitamin B12 absorption [1]. Effective vitamin B12 absorption requires three main conditions. The first condition is the formation of intrinsic factor by the gastric parietal cells [1]. The second condition is a healthy terminal ilium for absorption of the intrinsic factorvitamin B12 complex. And the third condition is availability of vitamin B12. Therefore, any pathology or surgical intervention that involves either the gastric parietal cells or the terminal ilium, or deficient sources of vitamin B12 in the diet, will invariably lead to vitamin B12 deficiency. However, the most common cause of vitamin B12 deficiency is an autoimmune gastritis [2] which destroys the parietal cells that produce the intrinsic factor. Other causes should be considered, particularly terminal ilium diseases, such as Crohn ’ s disease [3] and surgical resection from secondary malignancy. Sources of vitamin B12 are food derived from animals, with meats being an important source of vitamin B12 [1], Therefore, vegetarians are susceptible to B12 deficiency.
Vitamin B12 deficiency should be diagnosed promptly, because its replacement can cure most of the diseases related to its deficiency [1]. The systems most affected by vitamin B12 deficiency are the central and peripheral nervous systems, and bone marrow and its haematological products. Either of these systems can be affected in isolation or in combination. It is not uncommon for isolated, subacute combined degeneration of the spinal cord as first presentation of vitamin B12 deficiency, or isolated megaloblastic anaemia. Rare presentation of severe vitamin B12 deficiency is thromboembolic disease secondary to hyperhomocysteinemia. We present a rare complication of vitamin B12 deficiency, a massive pulmonary embolism.
Case Presentation
A 45-year-old mother of 5 children presented to her gynaecologist one month earlier with vaginal tears. This required uneventful posterior and anterior vaginal repair. The patient was discharged home in a healthy, normal status. She was mobilizing normally.Two weeks post-discharge, she presented to the emergency department with deep vein thrombosis, shortness of breath and central chest pain. A spiral chest CT angiography showed massive pulmonary embolism (Figure 1), with a filling defect in the main pulmonary artery and its branches, and increased pressure of the right ventricle, indicating acute pulmonary hypertension.
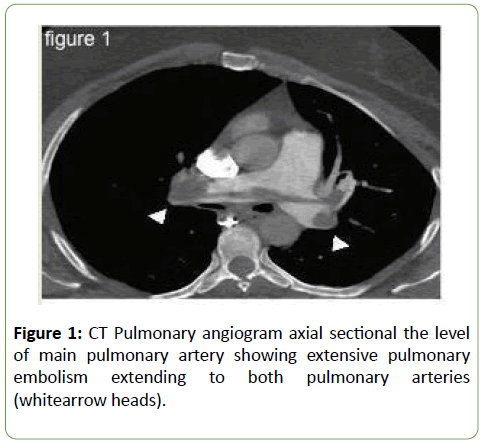
Figure 1: CT Pulmonary angiogram axial sectional the level of main pulmonary artery showing extensive pulmonary embolism extending to both pulmonary arteries (whitearrow heads).
Heparin was administered and a therapeutic coagulation profile obtained according to anticoagulation protocols and guidelines. The patient recovered and was discharged home on oral anticoagulation. During this presentation workup, a cause of the thromboembolic disorder was negative except crucially high serum homocysteine at 79.85 μmol/L (normal less than 15 μmol/L). Ten days post-discharge, the patient presented to neurosurgery with severe lower back pain and weakness and numbness in the lower limbs. At this time, neurological examination was significant for positive Romberg sign, including impaired proprioception in the lower limbs in both position and vibration. She had spastic tone and brisk deep tendon reflexes with extensor toe responses. Power decreased symmetrically at both upper and lower limbs at 4/5. Magnetic resonances images (MRI) of both the cervical and dorsal spine showed hyperintense lesions extending throughout the posterior cervical spinal cord on T2 MRI (Figure 2).
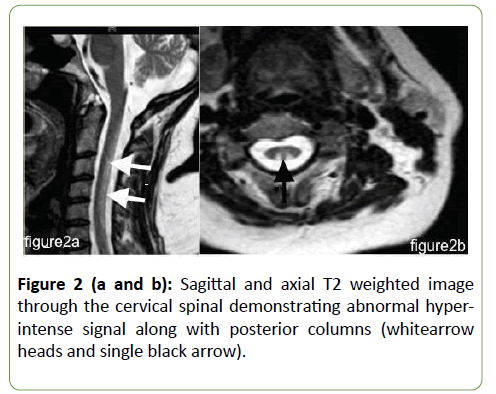
Figure 2 (a and b): Sagittal and axial T2 weighted image through the cervical spinal demonstrating abnormal hyperintense signal along with posterior columns (whitearrow heads and single black arrow).
Serum vitamin B12 was significantly low at 61 pmol/L (Normal 138–652 pmol/L); methylmelonic acid blood levels were also elevated. Further workup confirmed the diagnosis of pernicious anaemia. The patient was given parental vitamin B12, with normalization of both vitamin B12 and homocysteine blood levels. Clinically, the patient’s symptoms significantly improved and she was back to her previous level of activity (Figure 3).
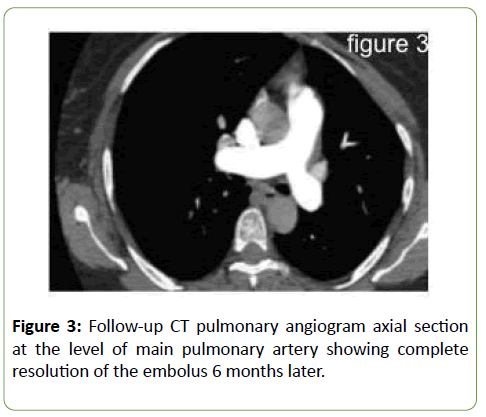
Figure 3: Follow-up CT pulmonary angiogram axial section at the level of main pulmonary artery showing complete resolution of the embolus 6 months later.
Discussion
LaRoy P. Penix reported in the Journal of Neurology the case of a 45-year-old lady with recurrent ischemic strokes and vitamin B12 deficiency secondary to hyper homocysteinemia[4]. In that case report, vitamin B12 parenteral replacement normalized her homocysteine and was presumed to protect her against additional strokes in the following year [4]. Thromboembolism in such cases is attributed to the injury of the vascular endothelium by the elevated blood levels of homocysteine [4]. In these patients, myocardial infarction, stroke and pulmonary embolism have been reported [5].
Metabolism of homocysteine requires three enzymes (cystathionine-beta synthase, methionine synthase and methyltransferases); these enzymes require vitamin B12, vitamin B6 and folic acid as co-enzymes [1]. Significant deficiency in any of these coenzymes will lead to poor homocysteine metabolism and the accumulation of homocysteine in the circulation. Additionally, subjects carrying the methylenetetrahydrofolatereductase (MTHFR677TT) genotype require higher amounts of folate and vitamin B12, which makes them even more at risk of arterial or venous thrombosis in the case of deficiency [6]. Clarke and colleagues in the New England Journal of Medicine showed that hyperhomocysteinemia is an independent risk factor for vascular disease [5]. In our case, there is no doubt that profound vitamin B12 deficiency contributed to the risk of deep venous thrombosis and the massive pulmonary embolism. In a prospective case control study, Perry et al. found that hyperhomocysteinemia is a strong and independent risk factor for stroke [7]. Martin Den Heijer and colleagues measured the plasma homocysteine levels in 269 patients diagnosed with deep-vein thrombosis and in 269 healthy controls matched to the patients ’ ages and sexes, concluding that high plasma homocysteine levels are strong factors related to deep-vein thrombosis in the general population [8]. Martinelli and others in a case control study of 121 patients with a first episode of cerebral vein thrombosis and 242 healthy control subjects found that hyperhomocysteinemia is associated with a 4-fold increased risk of cerebral venous thrombosis [9]. In a similar study,Morelli and colleagues found that fasting hyperhomocysteinemia is a risk factor for venous thrombosis [10]. It is clear that elevated serum homocysteine is an independent, predisposing risk factor for thromboembolic diseases in both arterial and venous vasculatures, atherosclerosis of the proximal large extra-cranial arteries, and most likely the cause of ischemic strokes in patients with hyperhomocysteinemia, which can be distributed in both the posterior and anterior circulation, indicating its embolic origin [4]. As mentioned above, the possible pathogenesis of venous thrombosis in hyperhomocysteinemia is not clear; however, hyperhomocysteinemia predisposes the venous endothelium to mechanical injuries, as a sequence of the initiation of a thrombus, with a subsequent cascade of hypercoagulopathy as more injuries continue. The repletion of the deficient coenzymes most likely will normalize the low vitamin B12, thuscorrecting the hyperhomocysteinemia [1]. This probably will prevent additional thromboembolic processes [4] or at least partially protect the patient from deep venous thrombosis and other cardiovascular events, given that the cause of these events has been removed. Thus, we believe, patients with deficient coenzymes, plus other risk factors for thromboembolism such as surgery, like our patient andatherosectrotic disorder, should be given supplementation of vitamin B12, vitamin B6 and folic acid.In a large prospective, population-based study, supplements combining folic acid, vitamin B12 and vitamin B6 did not reduce major cardiovascular events in patients with vascular disease [9].
Conclusions
• Low back pain is a rare presentation of vitamin B 12 deficiency.
• Vitamin B12 deficiency is a rare, but treatable, cause of venous thromboembolisms.
• More than one risk factor for a hypercoagulable state may lead to life threatening thromboembolism.
Acknowledgements
Dr. Hassan Ali Alayafi designed and obtained data, wrote the manuscript and reviewed the literature. Dr. Muhammad Ejaz Ahmad reviewed the radiological part, chooses the radiology images and wrote the legend of the figures.
28258
References
- Toh BH, Chan J, Kyaw T, Alderuccio F (2112) Cutting edge issues in autoimmune gastritis. Clin Rev Allergy Immunol 42: 269-278.
- Bermejo F, Algaba A, Guerra I, Chaparro M, De-La-Poza G, et al. (2013) Should we monitor vitamin B12 and folate levels in Crohn's disease patients?. Scand J Gastroenterol 48: 1272-1277.
- Penix LP (1998) Ischemic strokes secondary to vitamin B12 deficiency-induced hyperhomocystinemia. Neurology 51:622-624.
- Clarke R, Daly L, Robinson K, Naughten E, Cahalane S, et al. (1991) Hyperhomocysteinemia: An independent risk factor for vascular disease. N Engl J Med 324: 1149-1155.
- D’Angelo A, Coppola A, Madonna P, Fermo I, Pagano A, et al. (2000) The role of vitamin B12 in fasting hyperhomocysteinemia and its interaction with the homozygous C677T mutation of the methylene tetrahydrofolate reductase (MTHFR) gene. Thromb Haemost 83: 563-570.
- Perry IJ, Morris RW, Ebrahim SB, Shaper AG, Refsum H, et al. (1995) Prospective study of serum total homocysteine concentration and risk of stroke in middle-aged British men. Lancet 346: 1395-1398.
- Den Heijer M, Koster T, Blom HJ, Bos GM, Briët E, et al. (1996) Hyperhomocysteinemia as a risk factor for deep-vein thrombosis. N Engl J Med 334: 759-762.
- Martinelli I, Battaglioli T, Pedotti P, Cattaneo M, Mannucci PM (2003) Hyperhomocysteinemia in cerebral vein thrombosis. Blood 102: 1363-1366.
- Morelli VM, Lourenco DM, D'almeida V, Franco RF, Miranda F, et al. (2002) Hyperhomocysteinemia increases the risk of venous thrombosis independent of the C677T mutation of the methylenetetrahydrofolate reductase gene in selected Brazilian patients. Blood Coagul Fibrinolysis 13: 271-275.








