| Three dimensional conformal radiotherapy (3D CRT) is commonly used to treat breast cancer [1]. When breast alone is treated, 2 tangent beams are used. When regional nodes are included, 2 additional anterior-posterior beams are used – ‘4 field technique’ [2-4]. When internal mammary nodes are also included, wide tangent beams are used [5,6]. To achieve acceptable dose homogeneity, field-in-field technique is often employed – ‘forward planned intensity modulated radiotherapy (IMRT)’ [7]. Women with large body habitus present unique challenges for radiation planning with 3D CRT: large amounts of normal tissue (lung and heart) fall within irradiated volume; and dose heterogeneity is often unacceptable. These subject the patient to increased risk of radiation toxicity. Thus, we report our experience with a similar case where 3D CRT failed to meet planning objectives. In this article, we describe radiation planning with volumetric modulated arc therapy (VMAT), its associated challenges and strategies to overcome those challenges. |
Methods
|
|
Patient
|
| Our patient was a 49 year old female who underwent a modified radical mastectomy for a left breast cancer. The tumor was an invasive lobular carcinoma which was multifocal and multi-centeric (largest tumor measured 1.9 cm) with clear resection margins. It was positive for estrogen receptor (ER) and progesterone receptor (PR), and negative for human epidermal growth factor receptor 2 (Her2/neu). One sentinel node was positive; none of the 24 nodes removed at the time of axillary dissection were positive. She received 6 cycles of chemotherapy post surgery. After a discussion of the risks of local recurrence, benefits and side effects of radiation therapy, she elected for loco-regional radiotherapy. |
|
Simulation
|
| We simulated the patient on a breast board with both arms resting above head in arm and wrist supports. Prior to simulation, we placed three tattoos, outlined the chest wall with a wire and placed a 0.5 cm bolus on the chest-wall. We obtained freebreathing images on a 16-slice CT scanner (GE Healthcare, Salt Lake City, Utah) at 2.5 mm slice spacing; the scan range was from the level of the mastoid/ear lobe junction to 5.0 cm inferior to the most inferior extent of the ribs. |
| Using RPM system Varian® on the same CT scanner in breathhold mode, we obtained breath-hold CT images with same scan parameters. As per instructions, upon deep inspiration the patient held her breath for 20 seconds. |
| We subsequently transferred imaging data to the Eclipse® treatment planning system (TPS) for contouring of targets volume and organs-at-risk (OAR). |
|
Target volumes
|
| There were three clinical target volumes (CTVs). CTV50 comprised of the left chest wall. CTV45 comprised of level 2 and 3 left axilla. CTV40 comprised of the left internal mammary chain. We used RTOG atlas [8] to contour CTV50 and CTV45. For CTV40, we contoured the left internal mammary artery in 1-3 intercostal spaces; we expanded this volume by 1 cm at most (we excluded lung, muscle and bones from this volume). |
| Planning target volumes (PTVs) corresponded to a 5 mm geometric expansion on each CTV. Because PTV50 extended out of the patient’s body, for evaluation of dose volume histogram, we cropped PTV50_eval to lie within 5 mm of skin surface and called it PTV50_eval. |
|
Organs at risk (OAR) volumes
|
| OARs were left anterior descending artery (LAD), both lungs, contralateral breast, spinal cord, and heart (whole organ including great vessels). |
|
Dose prescription
|
| We prescribed 50 Gy in 25 fractions to PTV50_eval, 45 Gy in 25 fractions to PTV45, and 40 Gy in 25 fractions to PTV40. Our objectives were to achieve at least 95% dose coverage to PTV50_ eval and PTV45 and 100% coverage to PTV40; we accepted dose heterogeneity within 95% to 107% of the prescription dose. |
| For OARs, we defined dose constraints as follows: |
| Ipsilateral lung V5 < 50%, V20 < 40% |
| Mean lung dose < 18 Gy |
| Contralateral lung V20 < 15% |
| Spinal cord max dose < 40 Gy |
| Contralateral breast as low as possible |
| LAD dose max < 15 Gy and |
| Heart D5 < 500 cGy (dose to 5% heart less than 500 cGy) |
Technique
|
|
3D CRT
|
| We used modified wide tangents to encompass PTV50_eval and PTV40; lateral tangent had a gantry angle of 133° and the medial tangent had a gantry angle of 315°. To encompass PTV45, we used an anterior-posterior parallel opposed pair (POP). To keep dose off cord, we used an oblique angle for the anterior beam of the POP. Beam isocenter was located at the match line (junction of tangents and POP beams). Planning utilized 6 MV and 18 MV photons with field-in-field technique and three dose levels prescribed to the respective PTVs rather than to doses normalized to some prescription points. Field-in-field technique helps in achieving acceptable dose heterogeneity which ranges from 95% to 107% [9]. The use of alternate day bolus helps in achieving adequate dose coverage of PTV50_eval. |
| We repeated the same planning process on the breath hold scan. |
|
Volumetric modulated arc therapy
|
| For beam setup, we used two clockwise and counter clockwise 200o co-planar arcs within gantry angles of 140-300. The collimator angle setting was 30o for clockwise and 330o for counter clockwise arc. This was to reduce tongue-and-groove effect due to inter-leaf radiation leakage. The field size defined by X jaws were 15 cm to accommodate limited MLC leaf travel on a carriage within a single field. To ensure adequate incorporation of all three PTVs with respect to the jaw openings of the two arcs, we used the arc geometry tool of TPS. |
| Planning utilized RapidArc optimization product (version 10). The calculation grid size was 0.25 mm with heterogeneity correction. The forward dose calculation algorithm was Anisotropic Analytical Algorithm (AAA). The maximum dose rate was 600 MU/min and photon energy was 6 MV. Table 1 lists the dosimetric objectives. |
| For breath-hold VMAT planning, we used the same planning process with one exception. We used three sub-arcs in each of the clockwise and counter clockwise arcs. The reason for choosing sub-arcs was to circumvent the problem arising from treatment interruption if the patient was not able to hold her breath during arc therapy. When the treatment is interrupted, the gantry rotates back to its original start position. Given that the gantry travels 200o at the speed of 4.8 per second and the patient may comfortably hold the breath for 20 seconds, dividing each arc into three sub-arcs would allow delivery time of each arc to be less than 20 seconds. This should be well within the patient’s breath holding capacity. |
|
Chest wall motion
|
| We used fluoroscopy to assess chest wall motion. On a conventional simulator Ximatron (Varian, Palo Alto, CA), we obtained fluoroscopy images with the patient lying in a head-in first supine position. The beam energy was 70 KVp, and automatic exposure control (AEC) was enabled. The gantry angle was set at 90° such that the beam entered the left side of the patient. The patient continued to breathe normally during image acquisition. A video stream under the “simulation cine loop” acquisition type captured the fluoroscopy images of duration of 20 seconds at a frame rate of 2 images per second. |
| In addition, we fused breath hold scan to free breathing scan for slice by slice examination of chest wall motion. Breast board served as the reference region for fusion. |
|
Treatment delivery
|
| Varian iX linear accelerator that was equipped with a 120 Millennium MLC of 0.5 cm leaf width delivered the VMAT treatment. We used image guidance with daily KV-KV images. In addition, on the first three days of treatment, we obtained daily cone beam CT (CBCT) matched to chest wall and supraclavicular volume. When set up was deemed stable for three days, we obtained CBCT once every week. |
Results
|
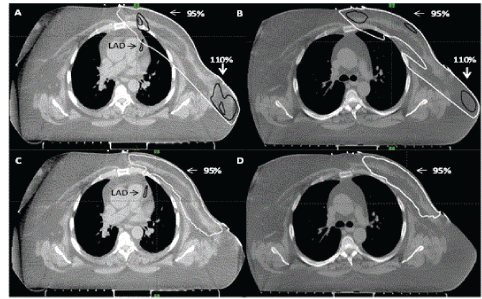
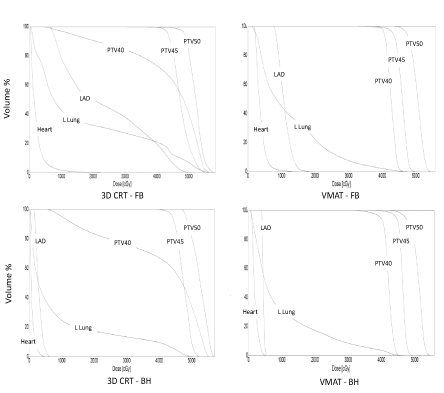
| In 3D CRT, 95% of PTV50_eval could be covered by 95% dose with hotspots of dose up to 115%. PTV40 could not be adequately covered because of excessive doses to the heart and lung. VMAT achieved planning objectives on target volumes and organs-atrisk. Fluoroscopy revealed minimal chest wall motion. Slice by slice examination of fused breath hold scan to free breathing scan revealed ≤ 5 mm chest wall motion. The heart and LAD moved away from the chest wall on deep inspiration (Figures 1 and 2). Comparison of the four techniques is shown in Table 2. |
Discussion
|
| To the best of our knowledge, this is the first report of the use of VMAT in loco-regional radiotherapy in a patient with large body habitus. In this left breast cancer post-mastectomy patient, 3D CRT failed to generate an acceptable and safe plan (even after excluding internal mammary nodes from the treatment volume). Dose heterogeneity was in the range of 95-116% in the chest wall. VMAT achieved acceptable dose homogeneity and it also achieved lower doses to organs at risk (coronary vessels, heart and lungs). |
| Our results are similar to those observed by others. Osman et al. [10] compared four techniques in 13 post lumpectomy patients with left breast cancer – 3D CRT with and without breath hold and VMAT with and without breath hold. VMAT plans had better dose conformity, target coverage, and reduction in dose to organs at risk (lung and heart) compared to 3D CRT plans. They observed further reduction in mean heart doses with VMAT breath hold technique compared to 3D CRT breath hold technique. |
| Popescu et al. [11] compared VMAT with IMRT and modified wide tangents for loco-regional treatment for left-sided breast cancer, including internal mammary nodes. They included 5 patients (post lumpectomy or post mastectomy) and each patient had 3 plans – one with each technique. Plans were dosimetrically compared. They concluded that VMAT achieved similar PTV coverage and sparing of organs at risk, with fewer monitor units and shorter delivery time than IMRT. However, low dose exposure to normal structures was higher in VMAT and IMRT compared to modified wide tangents. |
| Badakhshi et al. [12] compared VMAT with IMRT and 3D CRT in post lumpectomy setting. Out of 12 patients, 4 received loco-regional treatment. They did not find VMAT superior to the other techniques. Similarly, Jin et al. [13] compared five different radiotherapy techniques including VMAT and IMRT for left breast cancer in 20 patients. They did not recommend VMAT for left breast treatment. However, in both studies, dose coverage, dose homogeneity in VMAT was similar in comparison to 3D CRT. High dose volumes (V30, V40) for heart and lungs were lower in VMAT compared to 3D CRT. Low dose volumes (V5) were higher in VMAT compared to 3D CRT. Thus, in spite of different conclusions, our results were similar to Badakhshi et al and Jin et al. |
| Others dosimetrically evaluated VMAT for various applications in breast radiotherapy. Qiu et al. [14] compared VMAT with 3D CRT for partial breast radiation. Johansen et al. [15] compared VMAT with IMRT and 3D CRT for dose to contralateral breast. Nicolini et al. [16] compared VMAT with IMRT for bilateral breast irradiation. They all concluded that VMAT produced superior plans compared to IMRT and 3D CRT. |
| One issue with VMAT delivery in breath hold is that when treatment is interrupted because patient can no longer hold her breath, the gantry rotates back to its original start position. Osman and colleagues noted that in VMAT treatment delivery each arc had to be interrupted once or twice to allow the patient comfortably hold her breath [10]. Thus, each arc was subdivided into 4-6 sub arcs. For the gantry speed in our setting, we estimated that each arc had to be subdivided into 3 sub-arcs. |
| Another issue in VMAT is of chest wall motion. Since, VMAT plan is delivered through arc therapy, significant chest wall motion due to breathing may result in dose deposition outside the target volume. This is not the case with 3D CRT in which a 2 cm skin flash accounts for chest wall motion. Giorgia et al. [17] described a PTV expansion method for VMAT to account for chest wall motion. The method involves extending PTV outside the body surface; the region in extended PTV is assigned tissue equivalent Hounsfield unit. Our concern with this method was that high energy fluence would exist adjacent to body surface. This would result in excessive dose deposition to the surface when chest wall moves into the region of extended PTV. Thus, we performed fluoroscopy to assess chest wall motion. We also slice by slice examined the co-registered free breathing and breath hold scans. In our case, the chest wall motion was at most 5 mm. Hence, we delivered VMAT treatment in free breathing. |
| The major limitation of our study is that it is based on a single patient and thus the results may not be generalizable to every case. That said the main utility of VMAT in breast patients is in the setting of large habitus; in average built women 3D CRT or forward planned IMRT with or without breath hold achieves excellent dose coverage, dose homogeneity and dose constraints to organs at risk. Thus, our results should be used in the context of patients with large body habitus. |
| Another limitation is that this is a dosimetric study and clinical endpoints (local control, survival and toxicity) were not studied. Nevertheless, our patient did not experience any significant side effects such as skin burns, radiation pneumonitis, or pericarditis. We obtained a baseline CT coronary angiogram to monitor her risk of radiation induced coronary artery disease. |
Conclusion
|
| VMAT can produce superior dosimetric plans. When treatment with VMAT is being considered, chest wall motion must be carefully assessed using fluoroscopy and or a breath hold scan. When applicable, VMAT could be delivered in breath hold. This would not only further reduce the dose to the coronary vessels but it would also minimize chest wall motion, thereby improving precision of target delivery. |
Author’s Contribution
|
| WS and HX conceived the study. WS analyzed the data and wrote the paper. HX performed the VMAT plans and contributed in data analyses and in writing of the paper. Both authors approved the final version of the manuscript. |
Acknowledgments
|
| We acknowledge Nancy Sheaves and Lisa Sinclair for their help in performing free breathing and breath hold CT scans and 3D conformal radiotherapy planning, respectively, and Patricia Foley for her help in literature search. This work was supported in part by the Department of Medicine, Cape Breton Regional Hospital through a research grant #2014. |
Tables at a glance
|
 |
 |
| Table 1 |
Table 2 |
|
Figures at a glance
|
 |
 |
| Figure 1 |
Figure 2 |
|
| |
| |







