Review Article - (2024) Volume 12, Issue 1
What are the Different Cross Talks between HIF1-α, TGFβ, Integrin and ECM in Mediating Breast Cancer?
Sami Baccouche*,
Nejla Fourati,
Wissem Siala,
Rachid Jlidi and
Jamel Daoud
Department of Biology and Geology, Preparatory Institute to Engeneiring Study, Sfax, Tunisia
*Correspondence:
Sami Baccouche, Department of Biology and Geology, Preparatory Institute to Engeneiring Study, Sfax,
Tunisia,
Email:
Received: 14-Dec-2023, Manuscript No. IPACR-23-14367;
Editor assigned: 19-Dec-2023, Pre QC No. IPACR-23-14367 (PQ);
Reviewed: 02-Jan-2024, QC No. IPACR-23-14367;
Revised: 10-Jan-2024, Manuscript No. IPACR-23-14367 (R);
Published:
18-Jan-2024
Abstract
Extracellular Matrices (ECM) serve as the molecular scaffold for cell adhesion, migration, proliferation and differentiation and as a repository of cytokines and molecular cues that determine cell polarization and tissue organization. The EMC alteration conducts to Integrin β3 regroupement, after its TGFβ induced overexpression; important tumorogenesis step that mediates an amplified integrin-Fak-Src signaling leading to aggressive tumor phenotype.
The upstream of these events is the accumulation of stress sensor protein, HIF-1α, which in involved in a constitutive active TGFβ overexpression, epigenetic landscape remodeling and sustaining a Smad2/3 signaling by supressing VHL expression. Together, allowed TGFβ late target genes expression such as: SNAIL, MMP, galectin, paxillin and integrinβ3; and at the same time TGFβ early genes repression such as: p21 and E-cadherin.
This review highlight, subsequent to stress condition (as hypoxia), different feedbacks, whose HIF-1α, TGFβ, ECM alteration and integrin β3 interplay to promote breast cancerogenesis.
Keywords
TGFβ; Integrins β1/β3; HIF-1α; ECM; SMADs;
EMT; Menstrual phases swich; Src and breast
cancerogenesisa
Abbreviations
FAK: Focal Adhesion Kinase; Src: Nonreceptor
tyrosine kinases; Grb2: Growth factor receptorbound
protein 2, contains one SH2 domain and two SH3
domains; TET: A family of Ten-Eleven Translocation
(TET) methylcytosine dioxygenases; NF-κB: Nuclear Factorkappa
B; SOS: Son of Sevenless (SOS) a guanine nucleotide
exchange factors; Ras: Rat sarcoma protein, small GTPase or
small G-protein; Erk: Extracellular Signal-regulated
Kinases (ERKs) or classical MAP kinases; PI3K:
PhosphoInosotide 3 Kinase; AKT: Protein kinase B (PKB); Raf:
Rapidly Accelerated Fibrosarcoma: Serine/Threonine-
Specific Protein Kinase; Rac1: Member of Rho family
GTPase. MEK: Mitogen-activated protein kinase kinase also
known as MAP2K, MEK, MAPKK; ATF-2: Activating
Transcription Factor-2; CREB: CAMP-Responsive Element-
Binding Protein; Ets: E-twenty-six, Erythroblast Transformation Specific; Elk1: ETS Like-1 protein? P130Cas:
Breast Cancer Anti-oestrogen Resistance 1 (BCAR1) is a
member of the Cas (Crk-associated substrate) family of
adaptor proteins, DOCK: Dedicator of Cytokinesis: DOCK
family members contain a RhoGEF domain to function
as guanine nucleotide exchange factors to
promote GDP release and GTP binding to specific small
GTPases of the Rho family. YAP: Yes-Associated Protein,
transcription regulator by activating the transcription of
genes involved in cell proliferation and
suppressing apoptotic genes. STAT: Signal Transducer and
Activator of Transcription? PTP: Protein Tyrosine
Phosphatases, group of enzymes that remove phosphate
groups from phosphorylated tyrosine residues on proteins.
PELP-1: Proline-, glutamic acid- and leucine-rich protein 1
(PELP1) also known as modulator of non-genomic
activity of estrogen receptor (MNAR) and transcription
factor HMX3
Introduction
It’s evident that menstrual phases switch is dependent of
ovary hormones, oestrogen and progesterone. But when we
looked for their precise effect, we found that they impact firstly
on Extracellular Matrix (ECM) structure.
The impact of ECM on menstrual phases switch
Vogel and Coll, divided the morphologic variation of the
mammary gland, related to the menstrual cycle, into two major
phases: Proliferative phase (early phase) and secretory or
differentiated phase (late phase) which caracterized specifically
the behaviour of the normal epithial breast cell whitin the
menstrual cycle [1].
Extracellular Matrix (ECM) is a non-cellular three-dimensional
macromolecular network composed of collagens, proteoglycans/
glycosaminoglycans, elastin, fibronectin, laminins and several
other glycoproteins. Matrix components bind each other as well
as cell adhesion receptors forming a complex network into
which cells reside in all tissues and organs. Cell surface receptors
transduce signals into cells from ECM, which regulate diverse
cellular functions, such as survival, growth, migration and
differentiation and are vital for maintaining normal homeostasis.
ECM is a highly dynamic structural network that continuously undergoes remodeling mediated by several matrix-degrading
enzymes during normal and pathological conditions.
Deregulation of ECM composition and structure is associated
with the development and progression of several pathologic
conditions [2].
ECM of the mammary epithelium gland: The epithelium of
the mammary gland is composed of luminal cells, which line the
ducts and alveoli and myoepithelial cells which form the basal
cell layer that surrounds luminal cells and contacts the basement
membrane, a specialized form of ECM rich in collagen IV,
fibronectin, laminins and vitronectin [3].
Fibronectin (FN) serves as the molecular scaffold leading to
ECM contractibility. FN matrix assembly is a cell-mediated
process in which soluble dimeric. FN is converted into a fibrillar
network. Binding of cell surface integrin receptors to FN
converts it to an active form, which promotes fibril formation
through interactions with other cell-associated FN dimers
(Figure 1) [4].
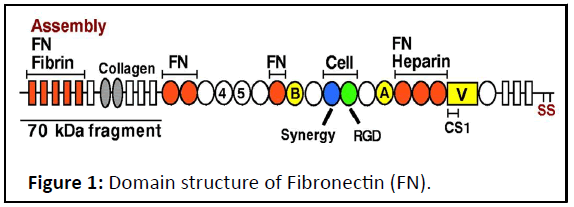
Figure 1: Domain structure of Fibronectin (FN).
FN consists of type I (rectangles), type II (ovals) and type III
(circles) repeats. Sets of repeats constitute binding domains for
fibrin, FN, collagen, cells and heparin, as indicated. The three
alternatively spliced segments, EIIIA, EIIIB and V (or IIICS), are in
yellow. The assembly domain and FN-binding sites are
highlighted in orange. SS indicates the C-terminal cysteines that
form the dimer. The RGD sequences (Arg-Gly-Asp) recognized by
integrin.
FN fibrils are not static but are rearranged and recycled by cell
movements, cell density and degradative processes [5]. This
elasticity provides a dynamic and pliable ECM environment to
accommodate cell activities within tissues and also provides the
potential for regulation of fibril organization and availability of
binding sites.
Literature Review
The impact of the cross talk between ERα, ECM and
integrin β3 on early phase
ERα promotes cell proliferation and inhibits the ECM effect: ERα, specific molecule of the early phase, is a ligand-dependent
transcription factor, across its transcriptional activating gene
expression propriety, ERα targets a variety of mitotic genes. In
fact, oestrogen, binding to ERα, induces its nuclear
translocation. Once in the nucleus, ERα induces the expression
of target genes, such as cyclin D1 and c-myc [6]. Also, among its
target gene, the Matrix-Metalloproteinase (MMP) [7]. Matrix
Metallopeptidases (MMPs), also known as matrix
metalloproteinases or matrixins, are metalloproteinases that are calcium-dependent zinc-containing endopeptidases; other
family members are adamalysins, serralysins and astacins [8].
Collectively, these enzymes are capable of degrading all kinds of
extracellular matrix proteins, essentially the different type of
collagen and fibronectin, but not vitronectin.
The deletion of fibronectin essentially in early phase, allows
vibronectin, becoming the major component of ECM, to interact
specifically to integrin β3 and initiating the integrin-FAK/Src
signaling pathway [9,10].
Integrin β3, a molecule of the early phase: Integrin
expression in the mammary epithelial cells is complicated as it is
regulated spatially and temporally as the gland develops and
through pregnancy, lactation and involution [11]. First,
mammary epithelial cells are anchorage dependent and require
cell-cell interactions or integrin-mediated attachment to the
ECM; in the absence of such adhesion, a cell will not proliferate
in response to growth factors and will succumb to a specialized
form of apoptosis-anoikis-that occurs as a result of detachment
from the E.C.M. Second, although integrin expression and
activation can vary within the gland, a somewhat limited set of
integrins are expressed-as assessed by immunohistochemistrywith
certain integrins restricted to either the luminal or
myoepithelial cells [12,13].
The β1 and β3 integrin subunits are expressed in epithelial cell
of the gland, while the β4 subunit exhibits a more restricted
expression pattern to myoepithelial cells [14]. Natural ligands of
integrins are component of the ECM such as vitronectin,
collagen or fibronectin. Thus, epithelial cells of the mammary
gland are capable of assembling at least eight functional integrin
receptors including two collagen receptors (α1β1 and α2β1),
three laminin receptors (α3β1, α6β1 and α6β4) and three
integrins (α5β1, αvβ1 and αvβ3) which recognize RGD
sequences (Arg-Gly-Asp) present in certain ECM molecule: αvβ1/
fibronectin and αvβ3/vitronectin. The repertoire of integrins
present at the membrane dictates therefore the extent to which
a cell will behave on a specific matrix and respond to its
environment. Once engaged with the ECM, integrins
heterodimer and recruit various signaling and adaptor proteins
to form focal adhesion complexes[14].
Integrin recognition of ECM proteins induces allosteric
changes that allow the receptor to transduce this signal across
the membrane, a process referred to as outside-in signaling.
Once engaged with the ECM, integrins heterodimer recruits
various signaling and adaptor proteins to form focal adhesion
complexes.
Integrin β3 involved in cell proliferation: The integrin β3
activation by the heterodimerisation of αv, after interaction with
specific EMC element, such as vibronectin, allows the binding of
ctyplasmic integrin domain to paxillin which recruits the FAK
protein (focal adhesion kinase) leading to its activation through
releasing the catalytic domain from FERM domain (Four-pointone,
Eszrin, Radixin, Moesi). Once the FERM domain inhibition
was arised, the kinase domain is autophosphrylated at Y397
tyrosine residue which provinding to its hyprphosphrylation in
certain tumoral circumstance (Figure 2). In fact, FAK is
scaffolding kinase protein, once activated, it recruits different types of proteins kinases whose their activation constitutes the
integrin β3-FAK transdution signals (Figures 3 and 4).
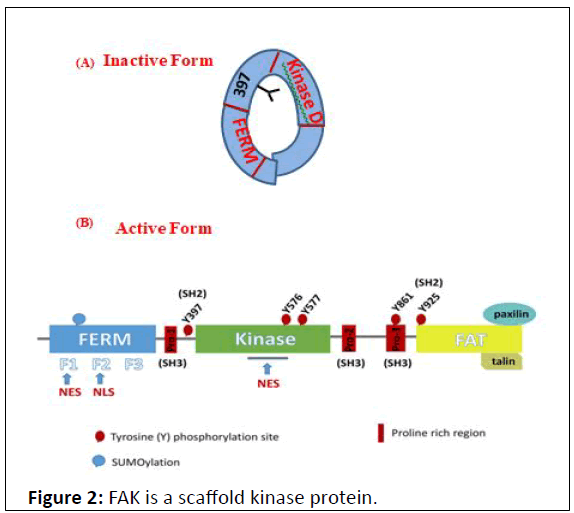
Figure 2: FAK is a scaffold kinase protein.
FERM is a kinase domain inhibiting. Binding with paxillin, the
inhibition set is arised, providing kinase domain to
autophosphrylation and potentially hyperphosphrylation at
different sites of catalytic domain; of wich FAK transduces the
integrin signaling pathway. Maximal FAK catalytic activation
occurs after Src mediated phosphorylation of FAK within the
kinase domain at Y576/577 and maximal Src activation occurs
after FAK phosphorylation of Src within the kinase domain at
Y419 [15].
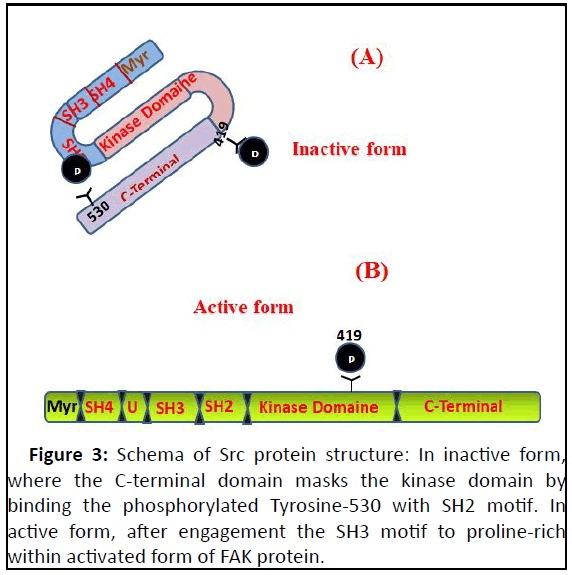
Figure 3: Schema of Src protein structure: In inactive form,
where the C-terminal domain masks the kinase domain by
binding the phosphorylated Tyrosine-530 with SH2 motif. In
active form, after engagement the SH3 motif to proline-rich
within activated form of FAK protein.
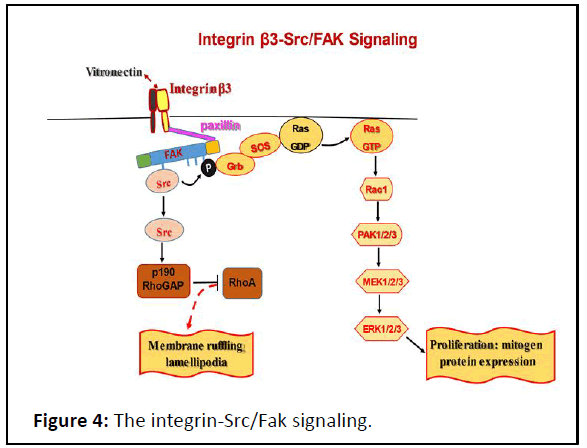
Figure 4: The integrin-Src/Fak signaling.
The activation of integrin β3 through its interaction to
vitronectin allowed the paxillin to interact with the cytoplasmic
tail domain of integrin β3. The binding FAT-domain of FAK to
paxillin arises the catalytic domain from FERM domain. Once the
FERM domain inhibition was arised, the kinase domain is
autophosphrylated at Y39 which undertakes Src to initiate two
downstream signals: Grb/Rac1 signaling leading to mitogen
protein expression enhancing cell prolferation and Src/
p190RhoGAP signaling leading to membrane ruffling
lamellipodia enhancing the morphogenesis.
Integrin β3 involved in morphogenesis: Cell motility is an
essential cellular process involved in numerous physiological
events including embryogenesis, wound healing, inflammation,
tissue regeneration and mammalian gland morphogeneis.
Motility is largely dependent on localized actin polymerization
at the leading edge of lamellipodia. The cell spreading and
motility share a common requirement for dynamic remodeling
of the actin cytoskeleton and focal adhesions through the
activation of Rho-family GTPase. In fact, the integrin-triggered
RhoA inhibition by p190RhoGAP enhances spreading and
migration by regulating cell protrusion and polarity (Figure 3).
The inhibition of RhoA activity that is induced transiently by
adhesion was antagonized by expression of dominant negative
p190RhoGAP [16].
The impact of the cross talk between PR-TGFβ, ECM
and integrin β1 on late phase
TGFβ a clue element of the late phase: The Transforming
Growth Factor-β (TGF-β) superfamily is distinct from other
cytokines owning to its more widespread and pleiotropic effects
[17]. A plethora of cellular activities, including cell proliferation,
differentiation, apoptosis, adhesion and migration, are
controlled by TGF-β superfamily members in a contextdependent
manner. Although cellular responses to TGF-β
signaling are mainly induced via its transcriptional regulation of
genes [18]. The TGF-β family consists of TGF-β1, 2 and 3 that
have largely redundant fnctions. Each isoform contains nine
highly conserved cysteine residues, mediating the formation of
inter or intramolecular disulfide bonds that interlock two TGF-β
polypeptides as a dimer. The dimeric TGF-β ligand associates with the pro-region-derived Latency-Associated Peptide (LAP)
and a Latent TGF-β Binding Protein (LTBP) and forms a Large
Latent Complex (LLC), which is trapped in the Extracellular
Matrix (ECM). Once activated, the dimeric TGF-β initiates
signaling by promoting the assembly of two type I (TβRI) and two
type II (TβRI) transmembrane receptors. Both of TβRI and TβRII
possess Ser/Thr kinase activity in the cytoplasmic domain.
Ligand binding results in the tetramer receptor complex
formation with two TβRI and two TβRII, in which TβRI is
activated via phosphorylation of Thr and Ser residues. The
phosphorylation-induced conformational change activates the
TβRI kinase that relays the signal to the effector Smad proteins
[19].
Upon activation of TβRI kinase activity, Smad2/3 is
phosphorylated at two serine residues and subsequently is
dissociated from the TβRI kinase domain, forming a trimeric
Smad complex composed of two Smad2/3 and one Smad4. This
Smad complex is then accumulated in the nucleus and acts as a
transcription factor to regulate contextual expression of target
genes through collaboration with diverse co-factors, as SP1.
In absence of stress conditions, the limit effect of TGFβ to
differentiation phase provided by VHL protein which mediates
Smad2/3 proteosome degradation; which attributed tumor
suppressor propriety to either TGFβ and VHL (Figure 5) [20].
TGF-β receptor-I and II expression was higher in stromal cells
than in epithelial cells during the secretory phase while no such
variation was observed during the proliferative phase.
Progesterone induces stromal decidualization indirectly, by
enhancing the expression and secretion of TGF-β from epithelial
cells [21]. The TGFβ involved in epithelial cell differentiation
process through two ways:
TGFβ remodels the ECM: In fact, TGFβ increases the
accumulation of the extracellular matrix proteins, fibronectin
and type I collagen. The increase in fibronectin and type I
collagen mRNA levels is an early response of cells to TGF-β [22].
TGFβ induces p21 and E-cadherin expression: TGFβ inhibits
cell cycle progression, in part through up-regulation of gene
expression of the p21WAF1/Cip1 (p21) cell cycle inhibitor. The
intracellular effectors of TGFβ, smad3 and smad4, functionally
cooperate with Sp1 to activate the human p21 promoter (Figure
5).
Smad proteins play important roles in regulation of the p21
gene by TGFβ and the functional cooperation of Smad proteins
with Sp1 involves the physical interaction of these two types of
transcription factors [23].
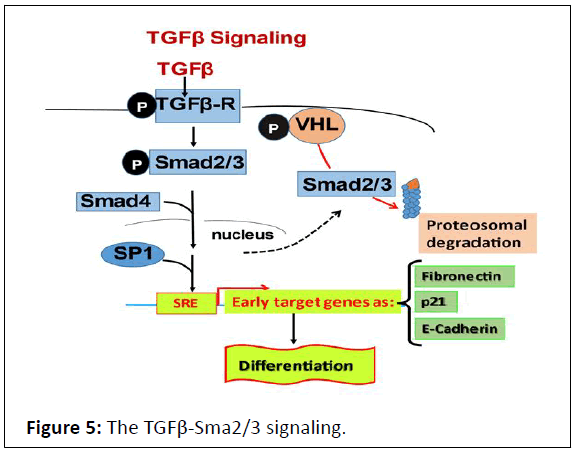
Figure 5: The TGFβ-Sma2/3 signaling.
Phosphorylation of TGF-receptor dimer is after TGFβ binding
and initiates smad2/3 signaling, where smad2/3 are
phosphorylated and recruits Smad4 to bind to SP1 transcription
factor in nucleus. The complex: Smad2/3-Samd4-SP1, interacts
with Smad-Reponsive-Element (SRE), promotor motif, to
activate transcription of target early genes involved in late
menstrual phase. The limit effect of this signling, on this
differentiation phase, is provided by pVHL Sma2/3 proteosomal
degradation.
In epithelial cells, E-cadherin-containing cell-to-cell junctions
are often adjacent to actin-containing filaments of the
cytoskeleton. The accumulation of E-cadherin-mediated
adherens junctions, the membrane cytoskeleton and the Na/
KATPase [24,25]. E-cadherin is involved in cell contact inhibition
of epithelial mesenchymatous transition. Loss of function
contributes to progression in cancer by increasing proliferation,
invasion and/or metastasis. The establishment of spatial
coordinates during the differentiation of polarized cells involves
a positional cue from cadherins. E-cadherin is expressed mainly
at the cell membrane after TGF-β stimulation [26].
Integrinβ1/fibronectin interaction mediates stress
fibers/actomyosinne contractibility, essential step to
proliferation inhibition and cell differentiation
induction
FN fibril elongation involves centripetal tensin-dependent
translocation of α5β1-integrin from focal adhesions along actin
stress fibers, forming fibrillar adhesions that promote
conformational changes in soluble FN dimers and assembly of a
fibrillar network [27]. During cell spreading, translocation of
ligand-occupied α5β1 integrins away from focal contacts and
along bundles of actin filaments generates ECM contacts. Which
enhanced by TGFβ induction fibronectin secretion by
fibfroblaste. Tensin is a primary cytoskeletal component of these
ECM contacts and a novel dominant-negative inhibitor of tensin
blocked ECM contact formation, integrin translocation and fibronectin fibrillogenesis without affecting focal contacts.
Whereas the vitronectin receptor αvβ3 remains within focal
contacts, the fibronectin receptor α5β1 translocates from focal
contacts into and along Extracellular Matrix (ECM) contacts [28].
As FN fibrils form on the outside of the cell, cytoplasmic
domains of integrin receptors organize cytoplasmic proteins into
functional complexes inside. Intracellular connections to the
actin cytoskeletal network and stimulation of certain key
intracellular signaling pathways are essential for FN-integrin
interactions and propagation of FN fibril formation. Thus,
assembly of native functional ECM depends on exquisite
coordination between extracellular events and intracellular
pathways (Figure 6).
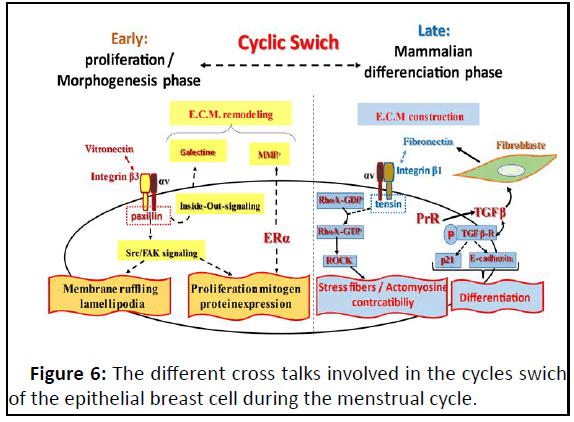
Figure 6: The different cross talks involved in the cycles swich
of the epithelial breast cell during the menstrual cycle.
The entering into the early cycle is initiated by the estadiol-
ERα binding which promotes the expression of ERα target genes
launching eitheir the cell cycle and the alteration of the
fibronectin-integrin β1 binding. This E.C.M. remodeling favors
the interaction of vitronectin-integrin β3 launching ouside-inintegrin
signaling leading to cell proliferation and membrane
ruffling and lamllipoda which facilited by the E.C.M remodeling
by the inside-out signaling. The late phase transition is
promoted by the fibronectin synthesis by TGFβ; the subsequent
inetgrinβ1-fibronectin interaction through the engagement of
tensin specifically leads to activate the outside-in integrin β1
signaling guiding to stress fibers and actomyosine contractibility
which in-turn participate in the E.C.M construction. TGFβ
induces also other early target genes: p21 and E-cadherin
allowing cell differentiation.
The impact of the dysregulation of these different
cross-talks on epithelial breast cell behaviour in
stress conditions (hypoxia)
Hif-1α, a stress sensor transcription factor: Hypoxia-Inducible
Factor-1α (HIF-1α) plays central roles in the hypoxia response. It
is highly expressed in multiple cancers. In fact, HIF-1 is the term
coined in 1993 by Gregg Semenza for a transcription complex
bound to a Hypoxia-Responsive Enhancer (HRE) lying 3’ to the
erythropoietin gene. Since then, the key components of the
HIF-1 system have been identified. HIF-1α overexpression has
been associated with an unfavorable prognosis in most cancers, as it activates genes that play a role in promoting cancer
metabolism, angiogenesis, invasion, maintenance of stem cell
pools, cellular differentiation, genetic instability and metastasis.
The HIF family comprises 3 functional nonredundant a
subunits, HIF-1α, -2α and -3α which form a heterodimer with
the HIF-1β subunit. HIF-1α and HIF-2α are the most studied
members of this family and have been thought to be largely
overlapping in their proto-oncogenic function [29].
The β subunit is constitutively expressed and is also involved
in xenobiotic responses. The α subunit protein is readily
detectable in cells cultured under low oxygen conditions and is
virtually undetectable in most cells under standard tissue culture
conditions due to rapid proteasomal destruction. In hypoxia, the
α subunit dimerises with a β subunit and translocates to the
nucleus. After dimerization, HIF-1α/HIF-1β bind E-box motifs. As
E-box is genome wide spread, make the HIF-1α a strong
powerful gene regulatory networks involved in cell
development, homeostasis and cellular behaviour. HIF-1α is
target for posttranslational modifications (Figure 7). These
modifications are related to metabolic stress, hypoxia, oxidative
stress, pH and oncogenic signaling; making HIF-1α a principal
sensor of stressful microenvironment.
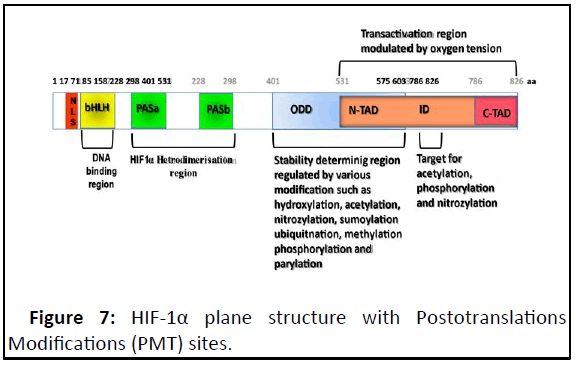
Figure 7: HIF-1α plane structure with Postotranslations
Modifications (PMT) sites.
The HIF-1α subunit has two Transactivation Domains (TAD):
NH2-terminal (N-TAD) and COOH-terminal (C-TAD). These two
domains are responsible for HIF-1α transcriptional activity. CTAD
interacts with co-activators such as CBP/p300 to modulate
gene transcription of HIF-1α under hypoxia. N-TAD is involved in
protein and DNA bindings. The Oxygen-Dependent Degradation
Domain (ODDD) overlapping N-TAD in their structures. This
ODDD domain is important in mediating O2 regulation stability.
Different types of PMT impacted on HIF-1α stability and on its
protein and DNA binding activities. The HIF-1α belongs to bHLHPAS
protein family, because their structures are related to two
nuclear proteins found in Drosophila (Per and Sim, PAS) which
have basic-helix-loop-helix (bHLH) motif. In general, the PAS
motifs are essential to allow heterodimer formation between
HIF-1α and HIF-1β subunits and b-HLH is essential for binding to
the HRE-DNA sequence on the target genes in the context of
permissive chromatin.
HIF-1α stabilized and accumulated in hypoxia
While hypoxia limits the proliferation of many cell types,
some cancer cells, stem/progenitor cells and pulmonary vascular
cells continue to grow and divide in low oxygen conditions.
Hypoxia is an important environmental stimulus that causes
genetic and metabolic reprogramming in cells to facilitate
survival. This programmed response is mediated primarily
through stabilization of Hypoxia-Inducible Factor 1α (HIF1α), a
transcription factor that coordinates a shift in energy
metabolism away from oxidative phosphorylation and toward
glycolysis and lactate fermentation through the increased
expression of Glucose Transporters (GLUT1), glycolytic enzymes,
Lactate Dehydrogenase (LDHA) and pyruvate dehydrogenase
kinase. In fact, in normaxi, HIF-1α is inactivated through
hydroxylation by HIF-Prolyl Hydroxylases (HPHs) also referred to
as Prolyl Hydroxylase Domain (PHD) proteins which form an
evolutionarily conserved subfamily of dioxygenases that uses
oxygen and 2-Oxoglutarate (2-OG) as co-substrates and iron and
ascorbate as cofactors [30].
The hydroxylation of the HIFa protein causes interaction with
the von Hippel Lindau (VHL) protein, a component of an E3
ubiquitin ligase complex. However, in the absence of oxygen the
PHDs that use oxygen in the hydroxylation reaction are inactive
and consequently HIF reaches a higher steady state level [31].
However, stability does not necessarily mean activity. In fact,
once HIF-1α is stabilized, it undergoes acetylation at 709 lysine
residue by p300 leading to its activation and enhacing its
stabilization. After p300 was autoacetylated in the same context
(Figure 8) [32].
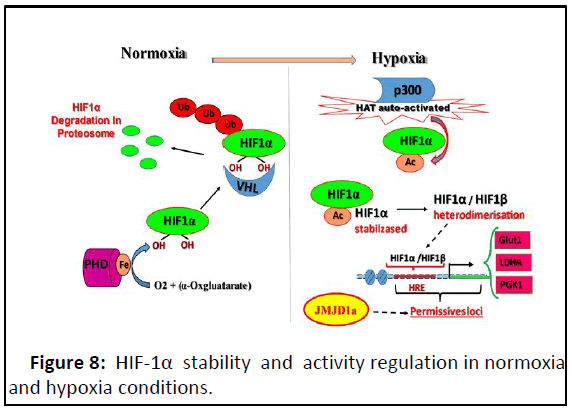
Figure 8: HIF-1α stability and activity regulation in normoxia
and hypoxia conditions.
In normoxia, PHD hydroxylates HIF-1α with Fe and α-
cetoglutarate as cofactors and leading to its degradation by
proteasomes proteins. In hypoxia, HIF-1α escapes from its
hydroxylation leading to its stabilisation but its transcriptional
activaty is achieved by p300 whose HAT activity is autoactivated
in hypoxia leading to the acetylation of HIF-1α. HIF-1α acetylated
heterodimerisases with HIF-1β; the heterodimee can bind to its
target consensus HRE sequences. But only permissive HREs were
accecibles, which are in open chromatin that mediated by KDMs
such as JMJD1a in this context.
G9A could participate in HIF-1α stabilization and up
regulation
G9A (KMT1C) methyltransferases is hypoxia-inducible at the
post translational level, strikingly similar to the regulation of
HIFa subunits (Figure 9) [33].
Given the hypoxia-inducibility of these KMTs, their extensive
enzyme-substrate networks, as well as the involvement and
regulation of their substrate proteins in hypoxia, the role of G9A
in hypoxia may be larger than what is currently known. In the
last decade, lysine methylation has been shown to participate in
the complex combinatorial PTM code that regulates HIF1α
function. HIF1α-K674 methylation was shown to occur in an
oxygen-independent manner and is associated with impaired
HIF-1α transcriptional activity.
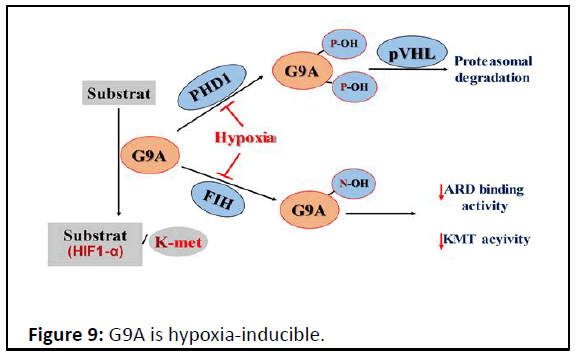
Figure 9: G9A is hypoxia-inducible.
G9A catalyzes lysine methylation of histone and non-histone
substrates. Like HIFa regulation, PHD1 induces prolyl
hydroxylation of G9A (P676 and P1207, occurring at higher
stoichiometry at the former proline) and pVHL-mediated
proteasomal degradation. FIH hydroxylated Ankyrin Repeat
Domains (ARDs) of G9A-N779 become impaired in both the
ability to bind mono- and dimethylated H3K9 products and the
hydroxylation inhibits di- and trimethylation of H3K9.
G9A could prepare an epigenetic landscape for HIF-1α gene
expression: A lot of data showed that under hypoxia stimulus the
HIF-1α stabilization is associated with HIF-1α gene overexpression.
In addition to the regulation of HIF-1α by protein stabilization,
several in vivo studies showed increased levels of HIF-1α mRNA
when mice, rats and ferrets were exposed to hypoxia. These
suggest that G9A, stabilized under hypoxia, could be involved in
displaying permissive chromatin in Hif-1α locus allowing
transcription factor such as the NF-kB, activated during hypoxia, to
trans activate HIF-1α gene leading to its over expression [34].
HIF-1 accumulation is associated with glycolysis
acceleration
Cancer cells undergo fundamental changes in their
metabolism to support rapid growth, adapt to limited oxygen
and nutrient resources and compete for these supplies with
surrounding normal cells. The lack of energy, which can result
from the absence of oxidative phosphorylation reactions, is
compensated by the high rate of glycolysis overproducing lactate, NAD+, polyolpathway and AGE. Glycolysis is the
production of the building blocks required for cancer
proliferation. In some cancer cells, a large proportion of glucose
is used in the serine de novo synthesis pathway, wherein 3-
phosphoglycerate is used by D-3-Phosphoglycerate
Dehydrogenase (PHGDH). Serine is also converted to glycine and
connected to the folic acid and methionine metabolism [35].
Thus, the serine biosynthesis pathway is also considered critical
for sustaining the growth of cancer cells (Figure 10).
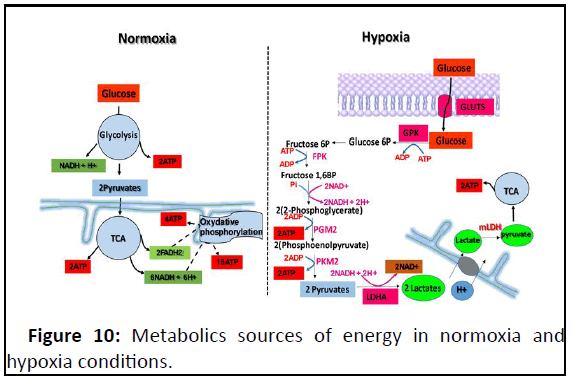
Figure 10: Metabolics sources of energy in normoxia and
hypoxia conditions.
The absence of oxidative phosphorylation chain in hypoxia
will be recompensed by glycolysis acceleration to approvision
maximum of energy for cell adaptation in that stressfull
condition (Figure 11).
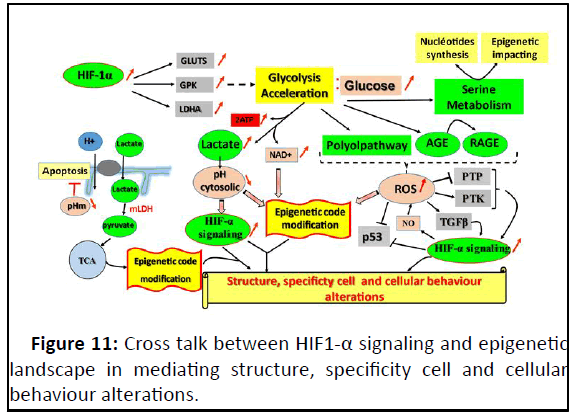
Figure 11: Cross talk between HIF1-α signaling and epigenetic
landscape in mediating structure, specificity cell and cellular
behaviour alterations.
HIF dependent glycolysis acceleration impacts on many
different cell metabolisms and generates ROS, metabolic sub
starts and cofactors with high availability; whose, each one can
impact on the epigenetic landscape and enhance different types
of HIF-1α signaling. Together affects the cell phenotype. These
HIF-1α impacts are on non-specific type of cell. What be can the
HIF1 impact on epithelial breast cell?
HIF-1α, via its dioxygenase enzymes inactivation,
inhibits VHL gene expression
A conserved role of pVHL in the regulation of TGF-β/SMAD3
signaling: Germline mutations of the VHL tumour suppressor gene cause von Hippel-Lindau (VHL) disease and are associated
with a high risk of early onset and multicentric clear cell renal
cell carcinoma. Somatic VHL gene mutations are found in
43%-57% of clear cell renal cell carcinoma cell lines and primary
tumours. pVHL, as an E3 ligase for SMAD3 ubiquitination,
directly interacts with conserved lysine and proline residues in
the MH2 domain of SMAD3, triggering degradation. As a result,
the level of pVHL expression negatively correlates with the
expression and activity of SMAD3 in cells, Drosophila wing and
patient tissues. In Drosophila, loss of pVHL leads to the upregulation
of TGF-β targets visible in a downward wing blade
phenotype, which is rescued by inhibition of Smad activity.
Drosophila pVHL expression exhibited ectopic veinlets and
reduced wing growth in a similar manner as upon loss of TGF-β/
SMAD signaling. Thus, our study demonstrates a conserved role
of pVHL in the regulation of TGF-β/SMAD3 signaling in human
cells and Drosophila wing development.
Many genes modified by promoter hypermethylation have
classic tumor-suppressor function. Example is the VHL gene in
renal cancer. 11%-19% of renal cell carcinoma cell lines and
primary tumours demonstrate promoter hypermethylation and
transcriptional silencing of the VHL gene.
Epigenetic VHL gene inactivation: The CpG islands of several
tumor suppressor genes acquire cancer-specific methylation and
many genes involved in familial forms of cancer undergo DNA
methylation-associated silencing in sporadic cancers [36]. These
changes are thought to contribute to uncontrolled proliferation
and thus tumor development. The Ten Eleven Translocation
protein 1 (TET1), a Fe/αcetoglutarate dioxygenase enzyme,
involved in DNA demethylation. TET1 functions to regulate the
lineage differentiation potential of embryonic stem cells [37].
TET1, as PHD, a Fe/αcetoglutarate dioxygenase enzyme, are
vulnerables indirectly or directly to pH cytosolic variations.
In fact, the glycolytically overproduced lactate is associated
with cytosolic acidification. A common feature of hypoxia, as
well as the tumor and stem cell microenvironments, is metabolic
acidosis [38]. One of the metabolic hallmarks of cancer is the
activation of glycolysis and lactate production. Furthermore,
lactate itself is used to further advantage by cancer cells. The
conversion of pyruvate to lactate regenerates the NAD+ cofactor
and contribute to cytosol acidification. The α-ketoglutarate (α-
KG), an intermediate of the Tricarboxylic Acid (TCA) cycle, is an
essential co-substrate for dioxygenases family due to its role in
Fe (II) coordination in the catalytic center. In fact, cytosolic
acidification moderately elevated 2-Hydroxyglutarate (2-HG) in
cells and boosting endogenous substrate TCA cycle intermediate
α-Ketoglutarate (α-KG) levels further stimulated this elevation.
pH can independently drive elevated 2-HG levels, pH regulation
of 2-HG may have important implications for 2-HG signaling in
hypoxia. The downstream signaling roles of D-2-HG in cancer
biology and of L-2-HG in hypoxia or stem cell biology are thought
to be mediated by epigenetic effects (Figure 12) [39].
The acccumulation of 2-Hydroxyglutarate (2-HG) inhibited
TET-dependent oxidation of 5 mC into 5 hmC in several cancers
including gliomas and hematological malignancies [40].
Directly pH can interfere with the catalytic site of dioxgenase
enzyme. In fact, the crystallographic studies on numerous
members of the Fe(II)/2OG-dependent oxygenase superfamily
have revealed two conserved structural features shared among
its members. First, the Fe (II) is ligated by two his residues and
(with the exception of the halogenases) a carboxylate from
either a Glu or an Asp residue; this metal-binding motif is
termed the 2-His-1- carboxylate facial triad. Second, the 2-His-1-
carboxylate motif is located within a Double-Stranded-Helix
(DSBH) fold, also known as the jelly-roll, cupin or jumonji C fold
[41].
Thus, the cytosolic pH acid, in modifying the charges of the
facial triad, according to pka of triad elements, alters the
catalytic activity, inactivating directly the α-KG-dependent
dioxygenase enzymes. Also, acidic pH is known to stabilize
Hypoxia-Inducible Factor (HIF): Through neutralisation function
of VHL by triggering its nucleolar sequestration and inhibition of
HIF Prolyl Hydroxylases (PHD) by 2-HG. Acidosis is involved in HIF
signaling feed back loop, that conducts to cell engagement to
irreversible malignancy phenotype [42].
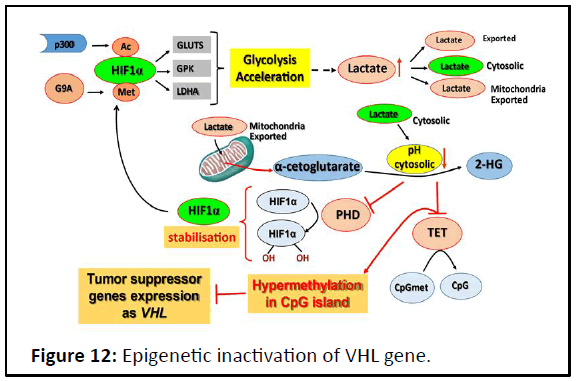
Figure 12: Epigenetic inactivation of VHL gene.
HIF-1α glycolysis acceleration induction conducts to pH
cytosolic acid which promotes FeII/αcetoglutarate dioxygenase
inactivation directly or through 2-HG production; causing an
hypermethylation in CpG island, mainly in tumor suppressor
gene promotors. Such as VHL.
The impact of the feed-back between HIF-1α and
ROS on TGFβ
ROS (Reactive Oxygen Species) are an intricate part of normal
cellular physiology. In excess, however, ROS can damage all three
major classes of macromolecules and compromise cell viability.
Accelerated glycolysis and ROS production: Through HIF-1α-
glycolysis acceleration inducing, different derivative
metabolisms can arise pathways releasing harmful reactive
oxygen species (Figure 13).
The cell metabolism of glucose excess can product metabolic
intermediates promoting unfavourable biochemical
consequences. Such metabolic intermediates: (1) sorbitol/polyol
and (2) hexosamine pathways; (3) augmented intracellular
formation of AGEs and expression of the Receptor for AGE (RAGE). These products are usually sources of intracellular
Reactive Oxygen Species (ROS) [43].
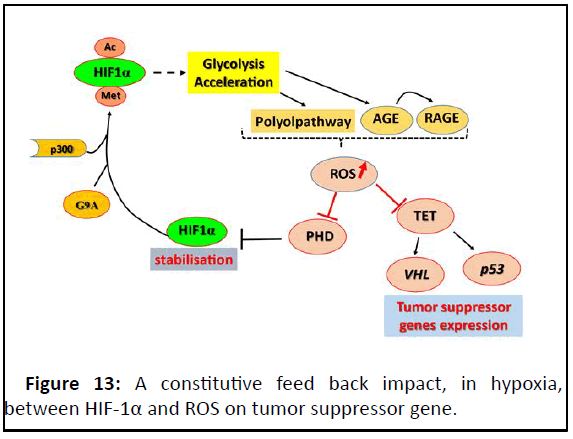
Figure 13: A constitutive feed back impact, in hypoxia,
between HIF-1α and ROS on tumor suppressor gene.
Feedback effect between NO synthesis and HIF-1α
NO radical is generated during the oxidation of L-arginine to Lcitrulline
by at least three different isoforms of the enzyme
Nitric Oxide Synthase (NOS). However, NO generated by the
inducible form of NO synthase (iNOS) has been implicated in
many pathophysiological states. Hypoxia causes an increase in
iNOS expression and that HIF-1 is essential for the hypoxic
regulation of iNOS gene expression. HIF-1 or a closely related
nuclear factor binds to the HIF-1 consensus sequence of the
iNOS promoter [44]. In otherwise, accumulation of NO, by
feedback, affect the stability and the level expression of HIF-1α.
In fact, NO transfer reactions between protein and peptide
cysteines have been proposed to represent regulated signaling
processes. Extensive biochemical and genetic data-including
both mutational analyses of Cysteine (Cys) residues in over 30
proteins that are targets of NO and creation of plants and mice
deficient in S-Nitrosothiol (SNO) metabolism-have led to the
current understanding that most actions of NOSs are in fact
conveyed by S-nitrosylation, the modification of protein Cys
thiols by NO [45,46].
Importantly, endogenous formation of NO in RCC4 cells via inducible NO synthase elicited S-nitrosation of HIF-1 alpha
leading to its stabilisation. All 15 free thiol groups found in
human HIF-1 alpha are subjected to S-nitrosation, as the ractive
Cys 800 [47]. NO can also inhibit PHD activity through
nitrosylation of cysteine residues or by binding the catalytic iron.
The ability of NO to bind the iron center of PHD appears to be
affected by the concentration of 2-OG, because inhibition is only
seen when 2-OG is unbound, indicating the metabolic status of
the cell can alter the effects of NO on HIF-1α stability [48].
NF-kB a knot of cross talk between ROS, HIF-1α and TGFβ
expression: NF-kB pathway signaling is a major regulator of
many important biological processes, including cell proliferation,
cell survival and elements of the immune response and is
considered a drug target for a range of pathologies including
inflammation and several kinds of cancer [49]. One of the NF-kB
signalling pathway targets is HIF-1α. In addition to the regulation of HIF-1α by protein stabilization, several in vivo studies showed
increased levels of HIF-1α mRNA when mice, rats and ferrets
were exposed to hypoxia [50].
In fact, HIF-1α encompassed in its promotor an NF-kB
responsive element. Interestingly, hypoxia induced Nuclear
Factor-kB (NFkB) nuclear translocation and activity. In line,
expression of the NFκB subunits p50 and p65 enhanced HIF-1α
mRNA levels, whereas blocking of NFkB by an inhibitor of
nuclear factor-kB attenuated HIF-1α mRNA induction by hypoxia.
In line, gel shift analysis and chromatin immunoprecipitation
confirmed binding of p50 and p65 NFkB subunits to the HIF-1α
promoter under hypoxia.
Reactive oxygen species directly link HIF-1α and NF-kB; ROSmediated
HIF-1α induction occurred on the transcriptional level
and was dependent on NF-kB. (NEMO), also known as IkB Kinase
γ (IKKγ), is a compelling but also a challenging target for drug
discovery. In complex with the catalytic IKKα and IKKβ proteins,
NEMO forms the active kinase IκB Kinase (IKK), which performs a
key role in activating NF-kB pathway signaling. IKK
phosphorylates IκB and triggers its proteolytic degradation,
allowing transcription factor NF-kB to translocate to the nucleus
where it modulates expression of biological effector genes.
Exposure to oxidizing conditions can induce NEMO homodimer
formation through disulfide bond formation between
Cys54-Cys54 and Cys347-Cys347. The disulfide-stabilized forms
of NEMO are active and that covalent dimerization preorganizes
the polypeptide in a conformation that confers relatively high
affinity binding with IKKβ. Thus, ROS, which are product of HIF
signalling, activate HIF-1 through PHD inhibition or S-nitrosation
of HIF-1α and induce its expression through NF-kB DNA binding
activation or NO radical accumulation. Marking an active loop
promoting a harmful weight impact on cell destination.
Feedback between ROS and TGF-β signaling
Noxs, an important ROS producers, is target of TGFβ
signaling: NAPDH oxidases (Noxs) are a group of hemecontaining
transmembrane proteins and important ROS
producers. Seven members have been identified in the Nox
family: Nox1, Nox2, Nox3, Nox4, Nox5, Dual oxidase1 (Duox1)
and Dual oxidase 2 (Duox2). TGF-β has been shown to induce
the expression of several Nox enzymes including Nox1, Nox2 and
Nox4 in different types of cells.
TGFβ is up regulated by ROS products: TGF-β is synthesized
and secreted into the extracellular space as a large latent
complex containing mature dimeric TGF-β bound to Latency-
Associated Protein (LAP). Release of TGF-β from LAP, a process
called latent TGF-β activation, is required for the binding of TGF-
β to its receptors. An increase in ROS after radiation exposure
leads to the activation of the TGF-β signaling pathway through
the oxidation of cysteine residues of the Latency-Associated
Peptide (LAP).
In fact, oxidation of LAP leads to a conformational change in
LAP, which allows the release of TGF-β from the latent complex;
it is known that ROS and TGF-β are interlinked by both feed
forward and feedback mechanisms. Numerous studies have
shown that ROS/RNS also up regulate TGF-β gene expression in various types of cells. TGF-β stimulation increases the basal level
of ROS through several NADPH oxidases (NOXs), including NOX4, via the canonical smad2/3 signaling factors.
In cultured human alveolar epithelial cells, xanthine/xanthine
oxidase derived ROS increased TGF-β1 production through a
transcriptional mechanism whereas S-nitroso-N-acetylpenicilamine
generated RNS induced TGF-β1 through
translational mechanisms. Nitrosylation of Cys162 in VHL
prevents it from ubiquitinating of either HIF-1α and Smad2/3.
Anette Teo et al. showed that, HIF-1 and Activator Protein 1
(AP-1) are involved in up regulation TGF-β expression, leading to
activation of the transcription factor Smad3 through autocrine
action.
We deduced that there are tree feedbacks: The one between
HIF-1α and ROS, the second between TGFβ and ROS and the
third is between HIF-1α/ROS and TGFβ; and these feedbacks are
interconnected and continuously active (Figure 14).
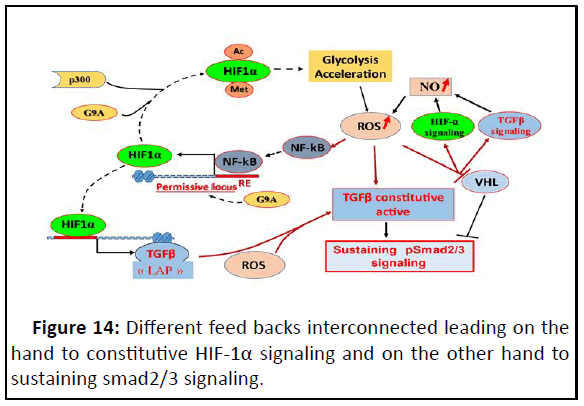
Figure 14: Different feed backs interconnected leading on the
hand to constitutive HIF-1α signaling and on the other hand to
sustaining smad2/3 signaling.
The HIF-1α epigenetic impact can overlap TGFβ
target genes (known as late genes) involved in
proliferation, EMT and cell migration
Chromatin is not static but changes according to the
regulatory cue including histone-modifying, histone
modification-recognizing and histone modification-erasing
proteins, so-called writer, reader and eraser proteins,
respectively. The structure of chromatin determines the
accessibility of DNA to transcriptional machinery; thus, it is
closely related to gene activity.
The epigenetic as defined by Andrian bird, the structural
adaptation of chromosomal regions so as to register, signal or
perpetuate altered activity states. Subsequent to HIF-1α
glycolysis acceleration induction, HIF-1α can remodels the
epigenetic landscape overlapping Smad responsive element
related to TGFβ target genes. These genes, called late genes, are
involved in proliferation, Epithelia Mesenchymal Transition
(EMT) and cell migration. Such as integrin β3, paxillin, snail,
MMP galectin and EMT markers etc. HIF-1α can remodels the
epigenetic landscape through many ways (Figure 15).
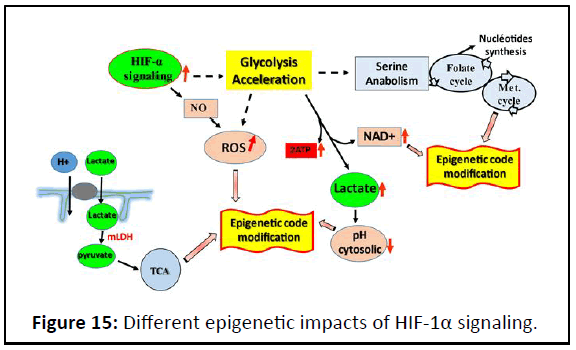
Figure 15: Different epigenetic impacts of HIF-1α signaling.
Serine anabolism: Consequently to high rate of glycolysis,
glycolytic intermediate 3-phosphoglycerate could be converted
to serine following three-step enzymatic reaction (Figure 16).
The serine, glycine, one-carbon network generates carbon units
that satisfy many metabolic demands including nucleotide
precursors for anabolic metabolism, redox maintenance and
substrates for methylation reactions that shape the epigenetic
landscape. Serine anabolism is coupled by two cycles
metabolism: Folate cycle associated with methionine cycle.
One metabolic intermediate of methionine cycle, S-adenosyl
Methionine (SAM). The methionine cycle provides methyl units
for a variety of reactions such as the methylation of proteins,
DNA, RNA and lipids, allowing for the modulation of their
biological functions (Figure 16).
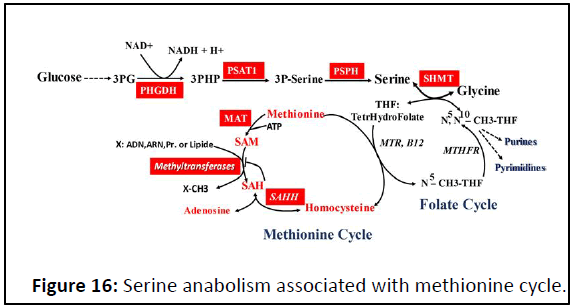
Figure 16: Serine anabolism associated with methionine cycle.
NAD+: NAD+ and its redox counterpart, NADH, are key
metabolites influencing a large constellation of metabolic
reactions. Nicotinamide Adenine Dinucleotide (NAD) is a coenzyme
that mediates redox reactions in various metabolic
pathways, including glycolysis, Tricarboxylic Acid (TCA) cycle,
oxidative phosphorylation and serine biosynthesis. There is an
abundance of data from model systems and humans that age
and conditions of metabolic stress challenge the NAD system in
affected tissues.
Continuous replenishment of NAD promotes the proliferation
and survival of fast-dividing cancer cells because elevated NAD
levels enhance glycolysis via Glyceraldehyde 3-Phosphate
Dehydrogenase (GAPDH) and Lactate Dehydrogenase (LDH) that
require NAD as a co-enzyme. PHGDH, a rate-limiting enzyme of
the serine biosynthesis pathway, also uses NAD as a co-enzyme
and the intracellular level of NAD is considered to be an
important regulator for serine biosynthesis in cancer cells.
Furthermore, NAD serves as a substrate for poly (ADP-ribose) polymerase (PARP) and sirtuins (NAD-dependent deacetylases)
and mediates poly-ADP-ribosylation and deacetylation,
respectively.
Therefore, the dynamic NAD+ and its metabolites levels, in
response to diverse cellular stress and physiological stimuli,
rewire biological processes via post-synthesis modification of
fundamental biomolecules, including DNA, RNA and proteins.
Acethyl-COA and epigenetic: The lactate passively diffuses
across the Mitochondrial Outer Membrane (MOM) into the
Mitochondrial Intermembrane Space (MIS). An increase in
lactate concentrations in the MIS facilitates conversion back into
pyruvate catalysed by an isoform of Lactate Dehydrogenase
(LDH) located in the mitochondria (mLDH). Pyruvate is then
shuttled across the Mitochondrial Inner Membrane (MIM) into
the matrix via a mitochondrial Monocarboxylate Transporter
(mMCT), where it is oxidized. The two reactions were near of the
equilibrium:

Thus, pyruvate is converted to Acetyl-coA, precursor of TCA,
leading, in the absence of OXOPHOS, to accumulation of either
acetyl-CoA, TCA intermediate metabolites and proton H+ intramitochondrial
(Figure 17).
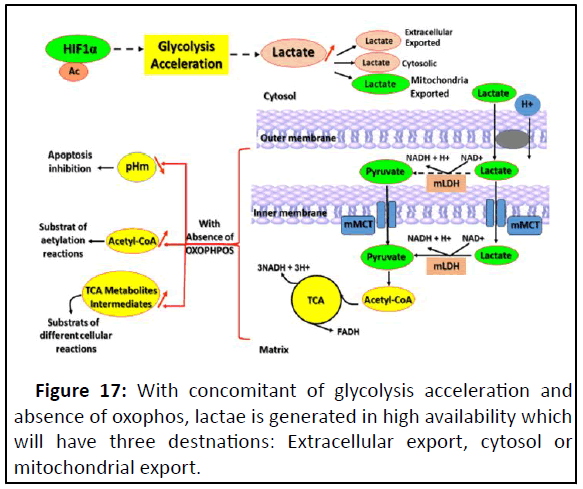
Figure 17: With concomitant of glycolysis acceleration and
absence of oxophos, lactae is generated in high availability which
will have three destnations: Extracellular export, cytosol or
mitochondrial export.
This figure focuses on that lactate mitochondrial export
leading to pH mitochondrial acid, affecting negatively the
apoptosis and high level of either acetyl-CoA or TCA metabolites
intermediates. There is a convention, that acetyl-CoA is not only
a central intermediate in the oxidation of glucose to produce
ATP, but also a precursor for the biosynthesis of numerous
metabolites required to build a new cell, such as lipids and
sterols.
It’s now admitted that, metabolites downstream of acetyl-CoA
could be signaling epigenetic modifications. In fact, Acetyl-CoA is
the principal acetyl donor for acetylation reactions within the
cell, essentially, which implicate Histone Acetyl Transferase (HAT) relies on intracellular levels of acetyl-CoA, that stands as a
prominent example of the interplay between metabolism and
chromatin dynamics. The acetylation of such a protein might
then enable it to perform some function required for growth or
proliferation.
pH cytosolic and epigenetic
Through 2-Hydroxyglutarate (2-HG) production, pH impact
on epigenetic landscape: In fact, cytosolic acidification
moderately elevated 2-Hydroxyglutarate (2-HG) in cells, and
boosting endogenous substrate TCA cycle intermediate α-
Ketoglutarate (α-KG) levels further stimulated this elevation. pH
can independently drive elevated 2-HG levels, pH regulation of
2-HG may have important implications for 2-HG signaling in
hypoxia.
The downstream signaling roles of D-2-HG in cancer biology
and of L-2-HG in hypoxia or stem cell biology are thought to be
mediated by epigenetic effects, because of competitive
inhibition of the α-KG-dependent dioxygenase superfamily of
enzymes. This includes the JmjC domain-containing histone
demethylases, The TET 5-methylcytosine hydroxylases and the
AlkB homolog family of DNA/RNA demethylases which can
inhibit DNA and histone demethylating enzymes resulting in the
glioma-CpG Island Phenotype (G-CIMP) and increased histone
methylation marks (Figure 18).
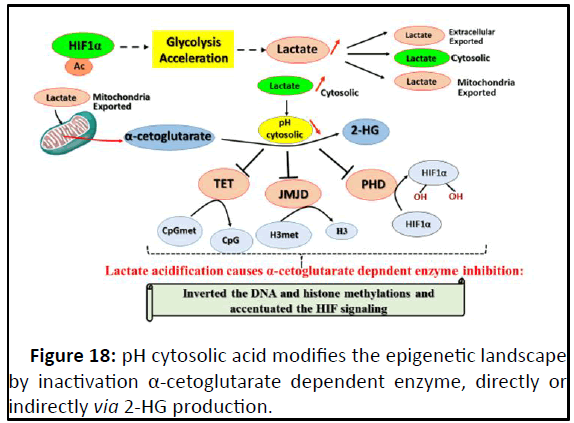
Figure 18: pH cytosolic acid modifies the epigenetic landscape
by inactivation α-cetoglutarate dependent enzyme, directly or
indirectly via 2-HG production.
Subsequent to lactate cytosolic availability, glycolysis
acceleration dependent, pH cytosolic becames acide leading to
2-HG formation and inhibiting three enzymes dioxygenases: TET,
JMJD and PHD, impacting thus on epigenetic landscape and
accentuating HIF-1α signaling.
ROS production and epigenetic
ROS affect TET protein activity: TET proteins contain a
carboxyl-terminal core catalytic domain that comprises a
conserved cysteine-rich domain and a Double Stranded β-Helix
domain (DSBH). Within the DSBH domain, there are key catalytic
residues that interact with Fe (II) and 2OG. Upon cofactor
binding, molecular oxygen oxidizes Fe (II) in the catalytic pocket,
thereby inducing the oxidative decarboxylation of 2OG and
substrate oxidation. TET proteins also have an additional domain that potentially regulates their chromatin targeting. At the
amino-terminal region, TET1 and TET3 have a DNA-binding
domain called the CXXC domain, which is composed of two
Cys4-type zinc finger motifs (Figure 19).
Redox regulation affects thiol posttranslational modificationaltering
molecule activity. In fact, in stressful condition, iterative
of ROS production, the thiol group within these different
domains of TET proteins could be undergone oxidative
modifications These include sulfenic (SOH), Sulfinic (SO2H) and
Sulfonic (SO3H) acids, disulfide bonds (PrSSPr) or nitrosothiols
(SNO). Such modifications can alter automatically the TET
protein activities such as TET-DNA-binding ability and α-KGdependent
dioxygenase activity.
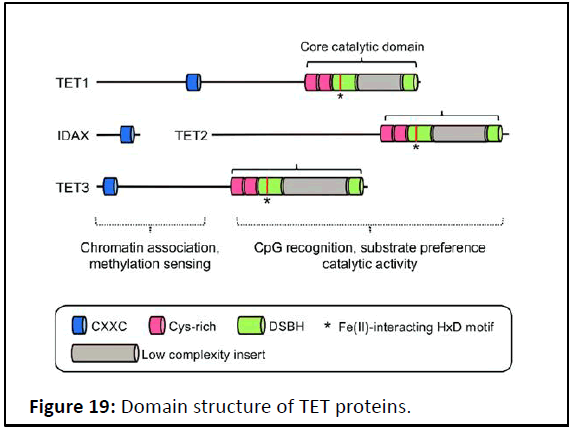
Figure 19: Domain structure of TET proteins.
The carboxyl-terminal core catalytic domain is highly
conserved among all TET family members and consists of a DSBH
domain and a Cysteine (Cys)-rich domain. The Cys-rich domain is
comprised of two subdomains and modulates the chromatin
targeting of TET proteins. The DSBH domain harbors key
catalytic motifs, including the HxD motif, which interacts with Fe
(II) and 2OG.
Discussion
The resultant of TGFβ overexpressed and
constitutive active is: A large spectre of TGFβ
permissive loci targets and sustaining smad2/3
signaling
The constitutive HIF-1α signaling, obtained through different
feed-back signaling, leads to constitutive active TGFβ
overexpression, enlarges the spectre of TGFβ target genes
encompassed the genes involved in proliferation, EMT and cell
migration. Such as: Integrin β3, MMPs, FAP, galectin, SNAILs
(SNAI1, SNAI2, ZEB1 and TWIST1, genes central to EMT.
And at the same time, in this same context, the sustaining
Smad2/3 signaling inhibits, in recruiting AP1 transcription factor,
the early genes involved cell differentiation such as p21 and Ecadherin.
Smad2/3 signaling recruits SP1 and AP1 to regulate TGFβ
target genes: Smads are the only substrate and signalling
transducers of the activated TGF-β-receptors. Nevertheless, the
positive and negative changes in the gene expression induced by
TGF-β signalling cannot occur with the Smad proteins only. Thus
Smad-dependent regulation of gene transcription is modulated
by the interaction with transcriptional co-activators or corepressors
(Figure 20).
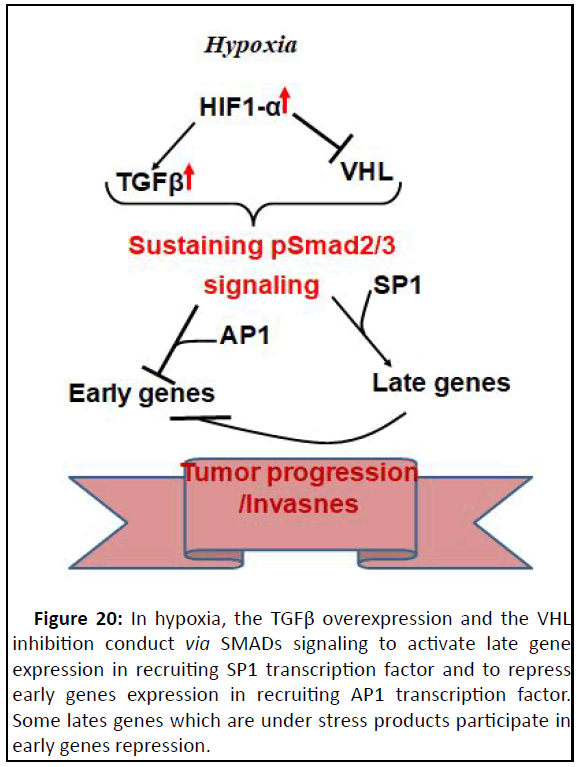
Figure 20: In hypoxia, the TGFβ overexpression and the VHL
inhibition conduct via SMADs signaling to activate late gene
expression in recruiting SP1 transcription factor and to repress
early genes expression in recruiting AP1 transcription factor.
Some lates genes which are under stress products participate in
early genes repression.
SP1 is involved in TGFβ late genes up regulation: SP1
(Specificity Protein 1) is a well-known member of a family of
transcription factors that also includes SP2, SP3 and SP4, which
are implicated in an ample variety of essential biological
processes and have been proven important in cell growth,
differentiation, apoptosis and carcinogenesis.
SP1 acts as a co-activator of the smad-dependent
transduction pathway. SP1 transcription factor is capable of
potentiating TGF-β-induced promoter activity of induced target
genes, through a physical interaction with smad3/smad4
complexes.
AP1 is recruited to repress TGFβ early target genes: A
plethora of physiological and pathological stimuli induce and
activate a group of DNA binding proteins that form AP-1 dimers.
These proteins include the Jun, Fos and ATF subgroups of
transcription factors. There is evidence that AP-1 proteins,
mostly those that belong to the Jun group, control cell life and
death through their ability to regulate the expression and
function of cell cycle regulators such as Cyclin D1, p53, p21cip1/ waf1, p19ARF and p16. Amongst the Jun proteins, cJun is unique
in its ability to positively regulate cell proliferation through the
repression of tumor suppressor gene expression and function,
and induction of cyclin D1 transcription. These actions are
antagonized by JunB, which up regulates tumor suppressor
genes and represses cyclin D1. An especially important target for
AP-1 effects on cell life and death is the tumor suppressor p53,
whose expression as well as transcriptional activity, are
modulated by AP-1 proteins.
The differential TGF-β regulation (transcription or repression)
between its various target genes depends on the context. The
remodelling of epigenetic landscape modulates the spectre of
permissive loci, for TGFβ regulation. The epigenetic context, as
well as recruitment of different cofactors of Smads, such as Sp1
or AP1, participate in this differentiation.
Up regulated TGFβ target genes
Integrin αVβ3: Integrin αVβ3 was highly expressed in tumors
than adjacent normal breast tissues. Over expression integrin
αVβ3 in tumors than adjacent normal breast tissues was an
indication of cancer progression with involvement of integrin
signaling. Integrin β3 is induced by TGF-β in A549 cells
(transformed cell line) to about 3.5 fold as compared to 1.8 fold
in HPL1D (untransformed cell line).
MMPS: Matrix Metallopeptidases (MMPs), are capable of
degrading the extracellular matrix proteins, essentially the
different type of collagen and fibronectin, but not vitronectin.
There is a considerable amount of evidence that Matrix
Metalloproteinases (MMPs) play an important role at different
steps of malignant tumor growth. The MMPs members family
are overexpressed in breast cancer tissue compared to normal
breast tissue. MMPs play an important role in the regulation of
EMT. Early studies showed that MMP3 directly degraded the
cell-cell adhesion receptor E-cadherin in mammary epithelial
cells leading to Epithelial Mesenchymal Transition (EMT). EMT is
a developmental process in which epithelial cells take on the
characteristics of invasive mesenchymal cells and activation of
EMT has been implicated in tumor progression. Recent findings
have implicated MMPs as promoters and mediators of
developmental and pathogenic EMT processes in the breast.
Many of these MMPs have also been associated with EMT
during cancer progression. However, a novel mechanism for
MMP-induced EMT involving TGF-β has been reported.
TGF-β has been shown to upregulate a number of matrix
Metalloproteinases (MMP) in epithelial cells, which may in turn
play a role in developing metastatic potential in these cells.
FAP: Fibroblast Activation Protein (FAP), a member of the
serine protease family, selectively expressed in the stromal
fibroblasts associated with epithelial cancers, whereas with low
or undetectable expression in the resting fibroblasts of normal
adult tissues. FAP was abundantly expressed in the stroma
across all breast cancer subtypes.
The Yixin Shi study further provided evidence that TGFβ
mediates up regulation of FAP expression in U87 glioma cells
through the canonical smad-dependent TGFβ signaling pathway,
in which activated TGFβ receptor induces phosphorylation of Smad (pSmad) and pSmad further directly activates
transcription of the FAP gene by binding to its promoter.
Galectin: Galectins as matricellular molecules regulate
integrin-mediated adhesion to the ECM. Galectins were
discovered through their galactoside binding activity, in a quest
to find proteins that decode complex cell-surface glycans.
They were defined as a protein family based on conserved
β-galactoside-binding sites found within their characteristic
~130 amino acid (aa) Carbohydrate Recognition Domains
(CRDs).
Galectin-9 is one of the crucial proteins used by various types
of cancer cells to suppress cytotoxic immune responses
and thus, escape immune surveillance. Some cancer cells
(Acute Myeloid Leukaemia (AML) and colorectal cancer) are
capable of secreting this protein, while other cancer cells
translocate galectin-9 onto the surface and use it to impair
anti-cancer activities of cytotoxic lymphoid cells such
as cytotoxic T lymphocytes and Natural Killer (NK) cells.
TGF-β1 expression, leading to activation of the transcription
factor Smad3 through autocrine action, triggers upregulation of
galectin-9 expression in both malignant (mainly in breast and
colorectal cancer as well as Acute Myeloid Leukaemia (AML))
and embryonic cells. The effect, however, was not observed in
mature non-transformed human cells.
SNAIL: Three SNAIL family proteins have been
identified in vertebrates: Snail1 (Snail), Snail2 (Slug) and
Snail3 (Smuc). All the family members encode transcriptional
repressors and share a similar organization with a highly
conserved C-terminal domain and bind to the E-box motif
in target gene promoters.
The TGF-β pathway is a master regulator of EMT due to its
ability to activate multiple transcriptional pathways that
ultimately coordinate to drive a cell towards a mesenchymal
phenotype. During TGF-β-induced EMT, SNAIL forms a
transcriptional repressor complex with Smad3/4. This complex
targets the adjacent E-boxes and Smad binding elements in
genes encoding junction proteins such as E-cadherin.
Moreover, loss E-cadherin correlated with nuclear coexpression
of snail1 and smad3/4 in a mouse model of breast
carcinoma and at the invasive fronts of human breast cancer. In
canonical TGF-β signaling, smad2/3 complexes with Smad4 to
regulate target gene expression, including SNAI1, SNAI2, ZEB1
and TWIST1, genes central to EMT.
The TGF-β pathway is a master regulator of EMT due to its
ability to activate multiple transcriptional pathways that
ultimately coordinate to drive a cell towards a mesenchymal
phenotype (Figure 21).
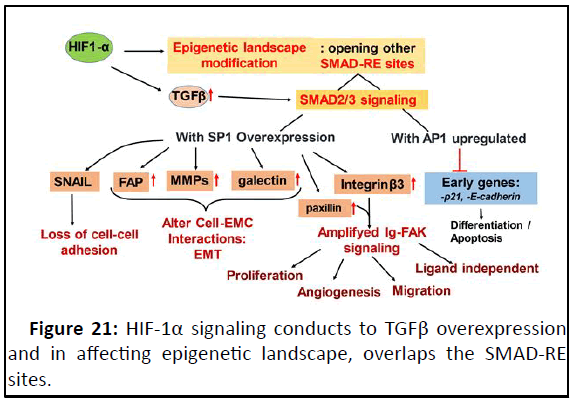
Figure 21: HIF-1α signaling conducts to TGFβ overexpression
and in affecting epigenetic landscape, overlaps the SMAD-RE
sites.
Sustaining smad2/3 signaling, thus, up regulates late genes in
recruiting SP1 factor and inhibits at the same time early genes in
recuiting AP1 factor. Subsequent to these signalings, the
epithelial breast cell undergoes different hallmark of tumor
behaviours leading to an aggressive tumor phenotype.
Via integrin β3/FAK signaling, TGFβ promotes
malignant tumor phenotype
Subsequent to proteases and galectin TGFβ induction, ECM
undergone structural alterations; essential step of promoting
outside-in signalings conducting to malignant cell tumor
phenotype. In fact, ECM alteration allowed the integrins
regroupement, the major element player of theses integrins is
integrin β3. This integrins regroupement enhances different
signaling pathways, arised from an amplified integrin-FAK-Src
signaling. Where much number of FAK proteins were
hyperphosphorylated downstream events of FAK/Src complex
formation. FAK overexpression is widely observed in numerous
tumor types and is used as a marker for invasion and metastasis. It
is highly overexpressed and activated in basal-like breast cancer.
FAK is a scaffold protein; depending on this propriety, it
activates, through its Kianse domain, a lot of factors that
transduce cell proliferation, survival and motility. Thus, the FAK
hyperphosphorylation, corresponding to the phosphorylation
sites between YP397 and YP925, activates different signaling
pathways (Figure 23). These phosphorylation sites serve as the
binding site for the SH2 domains of other proteins such as Src,
phosphatidylinositol-3-kinase (PI3K) and Grb2. The fully
activated Src-FAK complex phosphorylates other proteins,
including the adapter protein p130cas (Cas). Cas is
phosphorylated on multiple tyrosine residues by Src, which
forms binding sites for other signaling molecules bearing SH2
domains. Grb2 and p130cas (Cas) signalings are mediators
of cell proliferation, survival, migration and angiogenesis.
PI3K-AKT signaling: FAK activates the PI3K/AKT-mTOR.
Phosphorylation of PI3K activates AKT which regulates several
downstream molecules, including mTOR. NF-kB, is main
transcriptomic factor downstream of mTOR activation. As NF-kB target genes: HIF-1α, iNOS, COX2, MMP2/9 and bcl2. This
signaling pathway arises a constitutive active feed back between
TGFβ and HIF1α (Figures 22 and 23).
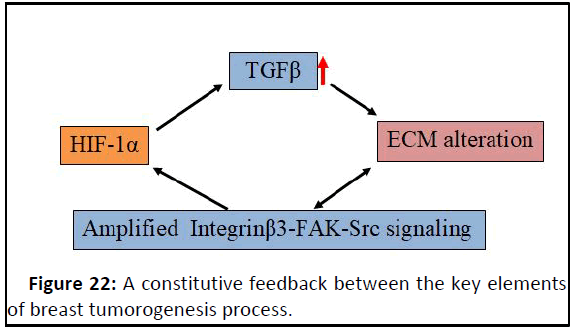
Figure 22: A constitutive feedback between the key elements
of breast tumorogenesis process.
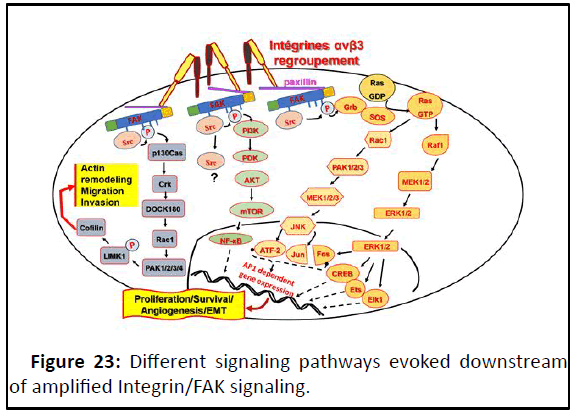
Figure 23: Different signaling pathways evoked downstream
of amplified Integrin/FAK signaling.
Src signaling: The proto-oncogene c-Src (Src) is a nonreceptor
tyrosine kinase whose expression and activity are correlated
with advanced malignancy and poor prognosis in a variety of
human cancers (Figure 24).
Breast cancer exhibits altered signal transduction pathways
involving Src. Evidence of increased Src activity and protein
expression levels has been found in human breast cancer tissue
relative to normal tissue.
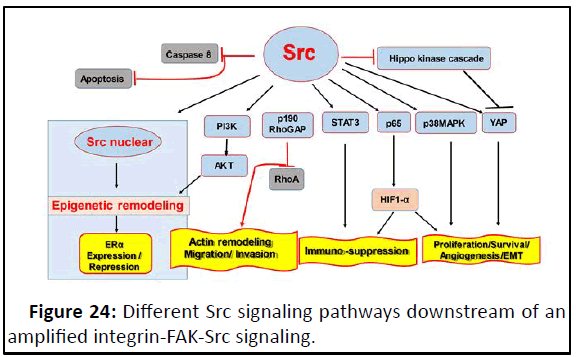
Figure 24: Different Src signaling pathways downstream of an
amplified integrin-FAK-Src signaling.
Src and motility: An early event upon integrin engagement is
the inactivation of the Rho GTPase in an Src-dependent manner.
The observation that Src can phosphorylate p190RhoGAP,
resulting in a decrease in GTP-binding capacity of Rho, provided
an early indication of a role for Src in the regulation of Rho. Src
promotes tyrosine phosphorylation of p190RhoGAP and
concomitantly, an activation of p190RhoGAP activity, which may
be responsible for the observed reduction in RhoA activity upon
cell adhesion.
Src and apoptosis: Caspase-8 is phosphorylated on amino
acid Tyr380 residue in a Src dependent manner whose
phosphorylation is very important for cell transformations and
enhanced by hypoxic conditions. The phosphorylation of Tyr380
residue of Caspase-8 may present a molecule switch to turn its
role from tumor suppressor to tumor activator. Caspase-8
represents the molecular switch that controls apoptosis,
necroptosis and pyroptosis and prevents tissue damage during
embryonic development and adulthood.
Src via epigenetic regulates ER gene expression: Beside
the membrane cytoplasmic function, Src has been
described in other subcellular compartments, as the
nucleus; where it’s involved in regulation of remodeling
epigentic enzyme activity.
Src via HDAC represses ER gene expression: The tumor
expression of Estrogen Receptors (ERs) is a very important
marker for prognosis and a marker that is predictive of response
to endocrine therapy. The loss of ER expression portends a poor
prognosis. This repression can be a result of the epigenetic
deacethylation mediated by histone deacethylase, HDAC or by
hypermethylation of CpG islands within the ER-α promoter. Src
was shown to phosphorylate and increase the activity of HDAC3.
Src may activate a transcrptional repressor to associate with
chromatin and/or alter its subcellular localisation.
DNMT1 has been found to interact physically with
either HDAC1 or HDAC2 through its N-terminus, thereby
forming a transcriptionally inactive chromatin structure that
represses transcription. Thus, DNA methylation and histone
deacetylation function through a common mechanistic
pathway to repress transcription.
Src via AKT regulates gene expression by targeting the
DNMT, HDMT and HMT activities: Histone phosphorylation that
depends on amino acids in histone is a dynamic process. Histone
phosphorylation occurs by altering many cellular processes,
including the cell cycle, repair of DNA damage and cell
apoptosis, so impaired regulation often leads to tumor
formation. Hence, the kinases that regulate the phosphorylation
of histones are always overexpressed in cancers.
In the same context, breast tumors over-express Src kinase
and AKT, also known as protein kinase B, is downstream of Src
effectors. AKT is linked to many of the cancer hallmarks and the
metastatic cascade in breast cancer. Up regulation of AKT in
cancer is associated with overall poor prognosis. AKT is
responsible for the phosphorylation of various epigenetic
regulators. Epigenetic regulators undergo extensive posttranslational
modifications, in particular, phosphorylation.
The Src/AKT pathway promotes transcriptional activation by
reducing global genome DNA methylation. This signaling
regulates DNMT1 through AKT-mediated phosphorylation at S143. The Src/AKT pathway favours transcriptional activation
through other means in addition to the reduction of H3K27me3.
Promoter associated H3K4me3 is characteristic of
transcriptionally active euchromatin and has been reported to
be elevated in breast and colorectal cancers, which are
commonly associated with Src-pathway activation. The Src/AKT
pathway was recently shown to be essential in regulating
H3K4me3 in in vivo models of AKT-activated breast cancer.
ROS mediates constitutive active Src
The Src tyrosine kinase and some of the members of its family
have been reported as redox regulated proteins. It has been
reported that Src tyrosine kinase undergoes oxidation/activation
in response to the formation of an S-S bond between Cys245
and Cys487, respectively located in the SH2 and in the kinase
domain of the Src molecule (Figure 25).
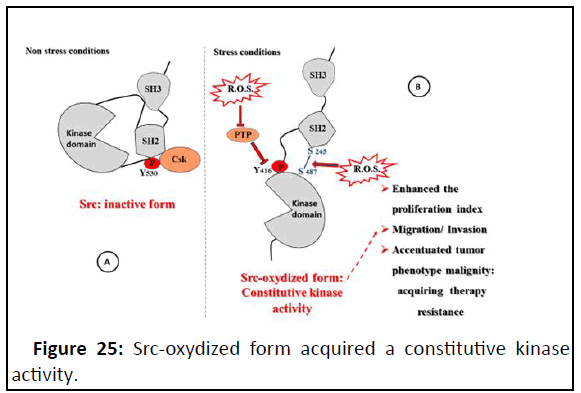
Figure 25: Src-oxydized form acquired a constitutive kinase
activity.
ROS induce Cys-245-Cys487 disulfide bond promoting the
release of Src tyrosine kinase (Csk) from the inhibitory tyrosine
530 residue of Src. This is followed by phosphorylation of the
tyrosine 419 residue in the activation loop of the Src kinase
domain.
Consequently, negative PTP oxidized/inhibited and activation
loop Tyr hyperphosphorylated extend the Src-mediated cell
proliferation to functional regulation of cytoskeletal
rearrangement and the acquirement of a spread cell shape for
anchorage dependent cells.
Src mediates hormono-therapy resistance
Src interferes with ERα and Her-2 signalings leading to
ligand-independent phenotype: ER, a ligand-dependent
transcription factor, has been implicated in the progression of
breast cancer; almost 70% of breast tumors are ER-positive at
the time of early diagnosis. ERα stimulates the expression of a
large number of protein involved in the regulation of the cell
cycle across binding to the consensus binding site: ERE or ERS
(Estrogen Responsive Element or Sites) in their promotors.
Which suggests that the overexpression of ERα favours the
overlapping binding sites, sharing some bases sequences
homology with ERE, even if with less affinity for expression other
oncogenes, whose involved in the proliferation/motility and antiapoptotic signals network. This context provides a further
regulatory function of ERα.
Independent of its ligand E2, the ERα function is also
regulated by phosphorylation through various kinase signaling
pathways that will impact various ERα functions including
chromatin interaction, coregulator recruitment and gene
expression, as well impact on breast tumor growth and on
breast cancer patient response to endocrine therapy.
AKT is recruited for ERα phosphorylation at specific sites,
pathway named as the cytoplasmic ERα signaling pathway. The
recruitment of PI3/AKT, from an amplified integrin-FAK-Src
signaling and/or from Src signaling, overlaps to ERα
phosphorylation, making ERα insensitive to its ligand described,
as a ligand-independent phenotype which is responsible for the
hormonal therapy resistance. By the same way, Src mediates the
phosphorylation of Her-2, activating, thus, the signal outcome
without its growth factor ligand, leading to ligand independent
tumor phenotype, such as resistant to Trastuzumab therapy
(Figure 26).
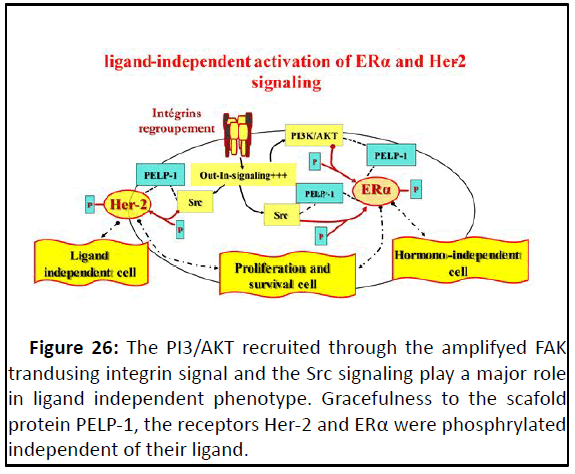
Figure 26: The PI3/AKT recruited through the amplifyed FAK
trandusing integrin signal and the Src signaling play a major role
in ligand independent phenotype. Gracefulness to the scafold
protein PELP-1, the receptors Her-2 and ERα were phosphrylated
independent of their ligand.
Conclusion
Through this study some remarks were arised:
• TGFβ plays dual contradictory roles, in normal condition, TGFβ
is involved in menstrual cyclic phases swich, in promoting
epithelial cell differentiation; but in stress condition (as
hypoxia) is involved in tumor promotion.
• In these two contexts, Smads are the only substrate and
signalling transducers of the activated TGF-β-receptors.
Nevertheless, the positive and negative changes in the gene
expression induced by TGF-β signalling cannot occur with the
Smad proteins only. Thus Smad-dependent regulation of gene
transcription is modulated by the interaction with
transcriptional co-activators (SP1) or co-repressors (Ap1).
• ECM remodeling constitutes essential step in menstrual cyclic
phases swich; also its alteration constitutes an essential
etiologic factors in tumorogeneis promotion.
• Also different feedbacks interconnected were highlighted,
whose the efficient therapy must take in consideration each
knot of this network.
Author's Contribution
Sami Baccouche conceived of the manuscript, performed and
wrote manuscript. The other authors read and approved the
final manuscript.
Ethics Approval and Consent to Participate
Not applicable here in this type of this study.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
References
- Vogel PM, Georgiade NG, Fetter BF, Vogel FS, McCarty Jr KS (1981) The correlation of histologic changes in the human breast with the menstrual cycle. Am J Pathol 104: 23
[Google Scholar] [PubMed]
- Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK (2016) Extracellular matrix structure. Adv Drug Deliv Rev 97: 4-27
[Crossref] [Google Scholar] [PubMed]
- Nelson CM, Bissell MJ (2006) Of extracellular matrix, scaffolds and signaling: Tissue architecture regulates development, homeostasis and cancer. Ann Rev Cell Dev Biol 22: 287-309
[Crossref] [Google Scholar] [PubMed]
- Wierzbicka-Patynowski I, Schwarzbauer JE (2003) The ins and outs of fibronectin matrix assembly. J Cell Sci 116: 3269-3276
[Crossref] [Google Scholar] [PubMed]
- Guan JL, Hynes RO (1990) Lymphoid cells recognize an alternatively spliced segment of fibronectin via the integrin receptor α4β1. Cell 60: 53-61
[Crossref] [Google Scholar] [PubMed]
- Lin CY, Strom A, Vega VB, Li Kong S, Li Yeo A, et al. (2004) Discovery of estrogen receptor α target genes and response elements in breast tumor cells. Gen Biol 5: 1-8
[Crossref] [Google Scholar] [PubMed]
- Verma RP, Hansch C (2007) Matrix Metalloproteinases (MMPs): Chemical-biological functions and (Q) SARs. Bioorg Med Chem 15: 2223-2268
[Crossref] [Google Scholar] [PubMed]
- Liu P, Sun M, Sader S (2006) Matrix metalloproteinases in cardiovascular disease. Can J Cardiol 22: 25B-30B
[Crossref] [Google Scholar] [PubMed]
- Lewis-Wambi JS, Jordan VC (2006) Treatment of postmenopausal breast cancer with Selective Estrogen Receptor Modulators (SERMs). Breast Disease 24: 93-105
[Crossref] [Google Scholar] [PubMed]
- Hynes RO (2002) Integrins: Bidirectional, allosteric signaling machines. Cell 110: 673-687
[Crossref] [Google Scholar] [PubMed]
- Taddei I, Faraldo MM, Teuliere J, Deugnier MA, Thiery JP, et al. (2003) Integrins in mammary gland development and differentiation of mammary epithelium. J Mammary Gland Biol Neoplasia 8: 383-394
[Crossref] [Google Scholar] [PubMed]
- Schwartz MA, Assoian RK (2001) Integrins and cell proliferation: Regulation of cyclin-dependent kinases via cytoplasmic signaling pathways. J Cell Sci 114: 2553-2560
[Crossref] [Google Scholar] [PubMed]
- Frisch SM, Ruoslahti E (1997) Integrins and anoikis. Curr Opin Cell Biol 9: 701-706
[Crossref] [Google Scholar] [PubMed]
- Shaw LM (1999) Integrin function in breast carcinoma progression. J Mammary Gland Biol Neoplasia 4: 367-376
[Crossref] [Google Scholar] [PubMed]
- Lim ST, Mikolon D, Dwayne GS, Schlaepfer DD (2008) FERM control of FAK function: Implications for cancer therapy. Cell Cycle 7: 2306
[Crossref] [Google Scholar] [PubMed]
- Arthur WT, Burridge K (2001) RhoA inactivation by p190RhoGAP regulates cell spreading and migration by promoting membrane protrusion and polarity. Molec Biol Cell 12: 2711-2720
[Crossref] [Google Scholar] [PubMed]
- Morikawa M, Derynck R, Miyazono K (2016) TGF-β and the TGF-β family: Context-dependent roles in cell and tissue physiology. Cold Spring Harbor Perspec Biol 8: a021873
[Crossref] [Google Scholar] [PubMed]
- Feng XH, Derynck R (2005) Specificity and versatility in TGF-β signaling through Smads. Ann Rev Cell Dev Biol 21: 659-693
[Crossref] [Google Scholar] [PubMed]
- Hata A, Chen YG (2016) TGF-β signaling from receptors to Smads. Cold Spring Harbor Perspec Biol 8: a022061
[Crossref] [Google Scholar] [PubMed]
- Zhou J, Dabiri Y, Gama-Brambila RA, Ghafoory S, Altinbay M, et al. (2021) pVHL-mediated SMAD3 degradation suppresses TGF-β signaling. J Cell Biol 221: e202012097
[Crossref] [Google Scholar] [PubMed]
- Kim MR, Park DW, Lee JH, Choi DS, Hwang KJ, et al. (2005) Progesterone-dependent release of transforming growth factor-beta1 from epithelial cells enhances the endometrial decidualization by turning on the Smad signalling in stromal cells. Molec Human Reprod 11: 801-808
[Crossref] [Google Scholar] [PubMed]
- Ignotz RA, Endo T, Massague J (1987) Regulation of fibronectin and type I collagen mRNA levels by transforming growth factor-beta. J Biol Chem 262: 6443-6446
[Google Scholar] [PubMed]
- Pardali K, Kurisaki A, Moren A, Ten Dijke P, Kardassis D, et al. (2000) Role of Smad proteins and transcription factor Sp1 in p21Waf1/Cip1 regulation by transforming growth factor-β. J Biol Chem 275: 29244-29256
[Crossref] [Google Scholar] [PubMed]
- Piepenhagen PA, Nelson WJ (1998) Biogenesis of polarized epithelial cells during kidney development in situ: Roles of E-cadherin-mediated cell-cell adhesion and membrane cytoskeleton organization. Molec Biol Cell 9:3161-3177
[Crossref] [Google Scholar] [PubMed]
- Nelson WJ, Hammerton RW (1989) A membrane-cytoskeletal complex containing Na+, K+-ATPase, ankyrin and fodrin in Madin-Darby Canine Kidney (MDCK) cells: Implications for the biogenesis of epithelial cell polarity. J Cell Biol 108: 893-902
[Crossref] [Google Scholar] [PubMed]
- Katuri V, Tang Y, Li C, Jogunoori W, Deng CX, et al. (2006) Critical interactions between TGF-β signaling/ELF and E-cadherin/β-catenin mediated tumor suppression. Oncogene 25: 1871-1886
[Crossref] [Google Scholar] [PubMed]
- Friedland JC, Lee MH, Boettiger D (2009) Mechanically activated integrin switch controls α5β1 function. Science 323: 642-644
[Crossref] [Google Scholar] [PubMed]
- Wang GL, Semenza GL (1993) General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Nat Acad Sci 90: 4304-4308
[Crossref] [Google Scholar] [PubMed]
- Keith B, Johnson RS, Simon MC (2012) HIF1α and HIF2α: Sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer 12: 9-22
[Crossref] [Google Scholar] [PubMed]
- Bertrand N, Castro DS, Guillemot F (2002) Proneural genes and the specification of neural cell types. Nat Rev Neurosci 3: 517-530
[Crossref] [Google Scholar] [PubMed]
- Chandel NS, Simon MC (2008) Hypoxia-inducible factor: Roles in development, physiology and disease. Cell Death Differ 15: 619-620
[Crossref] [Google Scholar] [PubMed]
- Stiehl DP, Fath DM, Liang D, Jiang Y, Sang N (2007) Histone deacetylase inhibitors synergize p300 autoacetylation that regulates its transactivation activity and complex formation. Cancer Res 67: 2256-2264
[Crossref] [Google Scholar] [PubMed]
- Liu S, Ye D, Guo W, Yu W, He Y, et al. (2015) G9A is essential for EMT-mediated metastasis and maintenance of cancer stem cell-like characters in head and neck squamous cell carcinoma. Oncotarget 6: 6887
[Crossref] [Google Scholar] [PubMed]
- BelAiba RS, Bonello S, Zahringer C, Schmidt S, Hess J, et al. (2007) Hypoxia up-regulates hypoxia-inducible factor-1α transcription by involving phosphatidylinositol 3-kinase and nuclear factor κB in pulmonary artery smooth muscle cells. Molec Biol Cell 18: 4691-4697
[Crossref] [Google Scholar] [PubMed]
- Locasale JW, Grassian AR, Melman T, Lyssiotis CA, Mattaini KR, et al. (2011) Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nature Genetics 43: 869-874
[Crossref] [Google Scholar] [PubMed]
- Weber M, Davies JJ, Wittig D, Oakeley EJ, Haase M, et al. (2005) Chromosome-wide and promoter-specific analyses identify sites of differential DNA methylation in normal and transformed human cells. Nat Gene 37: 853-862
[Crossref] [Google Scholar] [PubMed]
- Koh KP, Yabuuchi A, Rao S, Huang Y, Cunniff K, et al. (2011) Tet1 and Tet2 regulate 5-hydroxymethylcytosine production and cell lineage specification in mouse embryonic stem cells. Cell Stem Cell 8: 200-213
[Crossref] [Google Scholar] [PubMed]
- Phypers B, Pierce JT (2006) Lactate physiology in health and disease. British J Anaesth 6: 128-132
[Crossref] [Google Scholar]
- Aik WS, Chowdhury R, Clifton IJ, Hopkinson RJ, Leissing T, et al. Introduction to structural studies on 2-oxoglutarate-dependent oxygenases and related enzymes. Royal Society of Chemistry, Cambridge, 2015, pp. 59-94
- Nadtochiy SM, Schafer X, Fu D, Nehrke K, Munger J, et al. (2016) Acidic pH is a metabolic switch for 2-hydroxyglutarate generation and signaling. J Biol Chem 291: 20188-20197
[Crossref] [Google Scholar] [PubMed]
- Hegg EL, Jr LQ (1997) The 2‐His‐1‐carboxylate facial triad-an emerging structural motif in mononuclear non‐heme iron (II) enzymes. Eur J Biochem 250: 625-629
[Crossref] [Google Scholar] [PubMed]
- Mekhail K, Gunaratnam L, Bonicalzi ME, Lee S (2004) HIF activation by pH-dependent nucleolar sequestration of VHL. Nat Cell Biol 6: 642-647
[Crossref] [Google Scholar] [PubMed]
- Lacobini C, Vitale M, Pesce C, Pugliese G, Menini S (2021) Diabetic complications and oxidative stress: A 20-year voyage back in time and back to the future. Antioxidants 10: 727
[Crossref] [Google Scholar] [PubMed]
- Melillo G, Musso T, Sica A, Taylor LS, Cox GW, et al. (1995) A hypoxia-responsive element mediates a novel pathway of activation of the inducible nitric oxide synthase promoter. J Exper Med 182: 1683-1693
[Crossref] [Google Scholar] [PubMed]
- Sandau KB, Faus HG, Brune B (2000) Induction of hypoxia-inducible-factor 1 by nitric oxide is mediated via the PI 3K pathway. Biochem Biophys Res Communications 278: 263-267
[Crossref] [Google Scholar] [PubMed]
- Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS (2005) Protein S-nitrosylation: Purview and parameters. Nat Rev Mol Cell Biol 6: 150-166
[Crossref] [Google Scholar] [PubMed]
- Sumbayev VV, Budde A, Zhou J, Brune B (2003) HIF-1α protein as a target for S-nitrosation. FEBS Lett 535: 106-112
[Crossref] [Google Scholar] [PubMed]
- Chowdhury R, Flashman E, Mecinovic J, Kramer HB, Kessler BM, et al. (2011) Studies on the reaction of nitric oxide with the hypoxia-inducible factor prolyl hydroxylase domain 2 (EGLN1). J Molec Biol 410: 268-279
[Crossref] [Google Scholar] [PubMed]
- Dolcet X, Llobet D, Pallares J, Matias-Guiu X (2005) NF-κB in development and progression of human cancer. Virchows Archiv 446: 475-482
[Crossref] [Google Scholar] [PubMed]
- Bergeron M, Yu AY, Solway KE, Semenza GL, Sharp FR (1999) Induction of Hypoxia‐Inducible Factor‐1 (HIF‐1) and its target genes following focal ischaemia in rat brain. Eur J Neurosci 11: 4159-4170
[Crossref] [Google Scholar] [PubMed]
Citation: Baccoche S, Fourati N, Siala W, Jlidi R, Daoud J (2023) What are the Different Cross Talks between HIF1-?, TGF?, Integrin and ECM in
Mediating Breast Cancer? Archives Can Res, Vol.12 No.01: 001
































