Keywords
CADASIL – stroke – MRI
Introduction
Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leucoencephalopathy (CADASIL) is a hereditary disease of small arteries caused by different pathogenetic mutations of NOTCH-3 gene. It is characterized by the association of migraine with aura, psychiatric symptoms, recurrent ischaemic events at an early age and cognitive impairment. This and its typical radiological findings is what normally help us to suspect the disease. It is usually considered a disease of young and middle-aged adults, but cases in the elderly may be underdiagnosed. We report the case of a man who was diagnosed of CADASIL in his late seventies thanks to the previous diagnosis of his daughter.
Cases Reports
Case 1
Our patient’s daughter was 50 years old when she was sent to our hospital from the outpatient neurology clinic for further study, because of the radiological findings in her MRI scan of the brain, which consisted in white matter changes in both external capsules, bilateral periventricular areas and left temporal lobe.
She was attending the aforementioned clinic because she had suffered migraine with visual aura for 30 years, with some episodes suggesting visual aura without headache. She had no other symptoms. She was allergic to phosphomycin and had undergone tonsillectomy when she was a child. She had no other past medical history. Her family history was remarkable: her mother had migraine and mild dementia and her father had a stroke at the age of 75; her paternal grandfather, uncle and aunt had had strokes, and the uncle had been diagnosed of Alzheimer disease, a paternal cousin had received elsewhere the diagnosis of “ANA+ vasculitis” and another paternal cousin had been diagnosed of multiple sclerosis. She had two healthy sons. Physical examination, including a meticulous neurological exploration, was completely normal. The repeated MRI scan of the brain showed multiple hyperintense lesions in the white matter of posterior protuberance, periventricular and subcortical areas, including anterior temporal lobes, especially the left one, both centrum semiovale, internal and external capsules, with no signs of recent or past microbleeds (figure 1). All the other tests, including hemogram, glucemia, liver and renal function, erythrocyte sedimentation rate, serum thyroid hormones, hypercoagulability screening, vasculitis screening, EKG, chest x-ray, syphilis and HIV serologies, and supraaortic trunks ultrasonography with Doppler, were normal except for a cholesterolemia of 220 mg/dl with LDL cholesterol of 152 mg/dl. The skin biopsy was also normal. An Arg169Cys muta tion (cytosine to thymine substitution in the 505 codon) was found in the fourth exon of NOTCH 3 gene, which is known to be pathogenetic, so she was diagnosed of CADASIL. With this diagnosis, both parents were evaluated in our clinic.
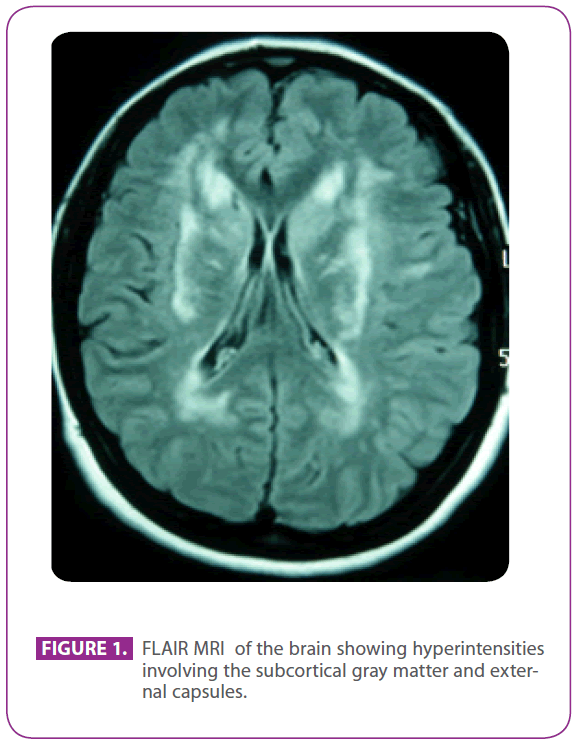
Figure 1: FLAIR MRI of the brain showing hyperintensities involving the subcortical gray matter and external capsules.
Case 2
Her mother was 78 years old. She had a long history of migraine without aura and, in the last two years, had developed a mild dementia with symmetric parkinsonism, fluctuations and REM sleep behaviour disorder, suggestive of dementia with Lewy bodies. Brain MRI ruled out relevant white matter disease.
Her father was a 79-years-old man with several vascular risk factors: diabetes mellitus type 2, dyslipidemia, and a smoking for 50 years, with an accumulated index of 15 pack-years, although he had quitted 3 years ago. He had ischemic heart disease with a posteroinferior myocardial infarction in 1991. In the last 8 years he had had three transient ischemic accidents and a lacunar stroke corresponding to different arterial territories, with progressive gait impairment, and in the last 6 months he had become a bit withdrawn and apathetic. He did not have a history of migraine. He was on metformin, clopidogrel, atenolol and atorvastatine. At physical examination he was conscious and oriented but a bit inattentive, language and remote and recent memory were normal, he showed ideomotor apraxia, altered similarity and sayings interpretation, with no frontal release reflexes. He also showed global hyperreflexia with bilateral flexor plantar reflexes, and a gait with short steps and reduced arm movements. A CT scan perfomed two years before, after one of his transient ischemic attacks , showed extensive white matter disease, especially prominent in the external capsule and temporal lobes (figure 2).
Summing up, the patient had recurrent ischemic strokes, mild subcortical cognitive impairment and leukoencephalopathy, highly suspicious of CADASIL considering his daughter´s diagnosis. A genetic test was performed, which showed the same NOTCH 3 mutation as her daughter and confirmed the diagnosis.
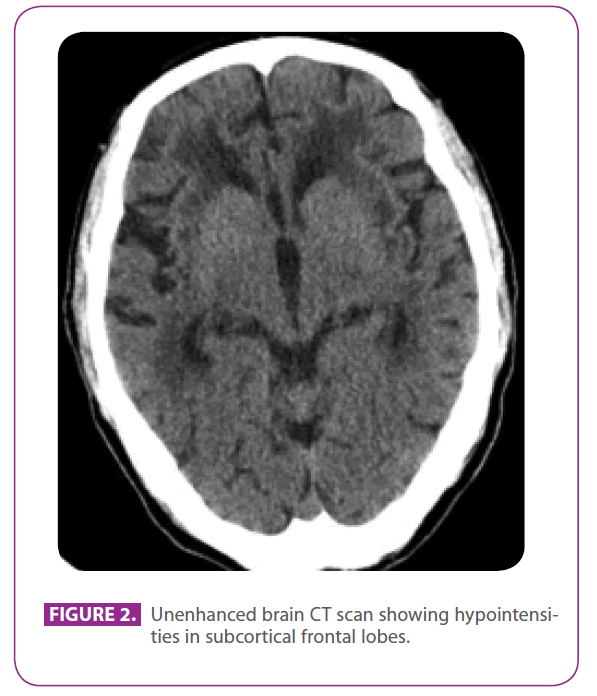
Figure 2: Unenhanced brain CT scan showing hypointensities in subcortical frontal lobes.
Discussion
CADASIL is the most common hereditary small vessel disease. However, it can also be sporadic, as de novo mutations have been described. It is caused by different pathogenic mutations in the NOTCH-3 gene, which is situated in chromosome 19, locus 19p13.2-p13.1, and consists of 33 exons which encode a 2321 aminoacids protein (1). This protein is a single-pass transmembrane cell surface receptor expressed in systemic arterial smooth muscle cells, with an extracellular regulator domain and an intracellular transductor domain. There are more than 190 mutations reported to date that can lead to CADASIL, and all of them occur in exons 2 to 24 of the NOTCH 3 gene, which encode the 34 epidermal grow factor-like repeats of the extracellular portion of NOTCH 3. Therefore, the screening of these 23 exons has 100% sensibility and almost the same specificity (2). Of these mutations, more than 180 are missense mutations, at least 6 deletions, one insertion, one frameshift and 2 duplications. Most of the pathogenetic NOTCH3 mutations occur in exons 3 and 4 (3).
All mutations result in an addition or loss of a cysteine residue in an EGF-like repeat, and therefore an odd number of cysteine residues that produces the formation of abnormal disulfide bridges. The mutant NOTCH3 causes degeneration of vascular smooth cells in small arteries and arterioles and accumulation of the abnormal protein in the wall of these vessels, leading to lumen stenosis (1). In the brain it affects penetrating cerebral and leptomeningeal arteries, provoking inability of these vessels to autorregulate and hypoperfusion of the territories irrigated by them, and therefore infarcts in the white matter.
The first symptom of CADASIL, when presenting, is usually migraine with aura, with an average age onset of 30 years. It appears in 20 to 40% of patients (4). Most attacks are typical with visual or sensory aura, but half of the patients have also atypical attacks with basilar, hemiplegic or prolonged aura.
Subcortical ischemic events, transitory attacks or strokes, appear in 60 to 85% of patients, the first one at a mean age of 50 years, although it can occur as soon as in the second decade. Most of the times there are no conventional vascular risk factors or they are not very important. In two thirds of the patients the ischemic events present clinically and radiologically as lacunar syndromes. Most of the patients have several strokes, usually 2 to 5, leading in several years to gait difficulties, urinary and fecal incontinence, dementia and pseudobulbar palsy (1). Cognitive impairment is the second most frequent clinical manifestation. The earliest sign is usually impairment in executive abilities and processing speed, which is present in most patients older than 35 years, but can appear as soon as at in the first decade of life (5). This cognitive impairment is progressive and normally worsens with recurrent strokes, with the addition of impairment in instrumental activities, memory, language, reasoning and visuospatial abilities. Over 70% of patients are demented by the sixth decade of life. Severe aphasia, apraxia or agnosia are rare in CADASIL.
Psychiatric disorders, mainly mood disturbances, appear in 20% of patients, in general as severe depressive episodes. Apathy is present in 40% of patients and does not relate with depression Other less common clinical manifestations are acute reversible encephalopathy (6) (in 10% of patients), most of them occurring after a migraine with aura, seizures (in 5 to 10% of patients), deafness, parkinsonism, cerebral hemorrhages (mostly in patients with high blood pressure), and myocardial infarction.
Despite its complete penetrance, CADASIL has an important inter and intrafamiliar clinical expression variability. The same NOTCH-3 mutation has a wide clinical spectrum, with no clear differences between homozygous and heterozygous patients. In fact, there is no correlation between genotype and clinical phenotype (4). The reason of these differences is not known, but some possible phenotype modifiers of CADASIL have been described, as current smoking for risk of stroke and age of the first stroke, high blood pressure for risk of stroke, or homocysteine levels for age of onset of migraine (3,4). These and other factors could influence the disease clinical expression by modifying the gene expression or by affecting other physiopathological routes that can lead to the same clinical manifestations.
Radiological changes appear in all individuals with a CADASIL mutation before 35 years old and increase with time. The earliest and most frequent features are hyperintense nonenhancing punctiform areas in cerebral white matter and subcortical structures in T2 weighted and fluid-attenuated inversion recovery images of MRI. Involvement of external capsules and the anterior part of temporal lobes is typical of this diseases and a clue for the diagnosis, as they help in the differential diagnosis with other conditions such as small-vessel disease (1).
CADASIL is commonly considered a disease of young and middle- age adults, but the number of elderly patients could be underdiagnosed (7,8). Our second case may serve as an example: a 79 year-old patient with vascular risk factors and recurrent strokes, with white matter disease that was considered secondary to small-vessel disease by several neurologist. The diagnosis was reached only after the knowledge of his daughter mutation. In cases without clear family history , an open mind and careful attention to suggestive radiological findings like the involvement of the external capsule or anterior temporal lobes may be the only clue for a correct diagnosis.
2207
References
- Chabriat H, Joutel A, Dichgans M, Tournier-Lasserve E, Bousser MG. Cadasil. Lancet Neurol 2009; 8:643-53.
- Vazquez do Campo R, Morales-Vidal S, Randolph C, Chadwick L, Biller J. CADASIL: a case series of 11 patients. Rev Neurol. 2011; 52:202-10.
- Adib-Samii P, Brice G, Martin RJ, Markus HS. Clinical spectrum of CADASIL and the effect of cardiovascular risk factors on phenotype: study in 200 consecutively recruited individuals. Stroke 2010; 4:630-4.
- Singhal S, Bevan S, Barrick T, Rich P, Markus HS. The influence of genetic and cardiovascular risk factors on the CADASIL phenotype. Brain. 2004; 127:2031-8.
- Dichgans M, Markus HS, Salloway S, Verkkoniemi A, Moline M, Wang Q, Posner H, Chabriat HS. Donepezil in patients with subcortical vascular cognitive impairment: a randomised double-blind trial in CADASIL. Lancet Neurol. 2008;7:310-8.
- Schon F, Martin RJ, Prevett M, Clough C, Enevoldson TP, Markus HS. “CADASIL coma”: an underdiagnosed acute encephalopathy. J Neurol Neurosurg Psychiatry. 2003;74:249-52.
- Liem MK, Lesnik Oberstein SA, Vollebregt MJ, Middelkoop HA, van der Grond J, Helderman-van den Enden AT. Homozygosity for a NOTCH3 mutation in a 65-year-old CADASIL patient with mild symptoms: a family report. J Neurol. 2008 ;255:1978-80.
- Lee YC, Yang AH, Soong BW. The remarkably variable expressivity of CADASIL: report of a minimally symptomatic man at an advanced age. J Neurol. 2009;256:1026-7







