Research Article - (2024) Volume 0, Issue 0
Zinc Supplementation in the Management of Acute Diarrhea in High-Income Countries – A Systematic Evaluation and Meta-Analysis
Túlio Revoredo1,
Lucas Victor Alves2,
David Romeiro Victor3 and
JoãoGuilherme Bezerra Alves4*
1Department of information retrieval, Institute of Integral Medicine Prof. Fernando Figueira (IMIP), Recife, Brazil
2Department of Neuropediatric, , Institute of Integral Medicine Prof. Fernando Figueira (IMIP), Recife, Brazil
3Faculty of Medical Sciences, University from Pernambuco, Recife, Brazil
4Department of Pediatrics, Institute of Integral Medicine Prof. Fernando Figueira (IMIP), Recife, Brazil
*Correspondence:
JoãoGuilherme Bezerra Alves, Department of Pediatrics, Institute of Integral Medicine Prof. Fernando Figueira (IMIP),
Brazil,
Email:
Received: 23-Jul-2024, Manuscript No. IPHSJ-24-15061;
Editor assigned: 25-Jul-2024, Pre QC No. IPHSJ-24-15061 (PQ);
Reviewed: 08-Aug-2024, QC No. IPHSJ-24-15061 (QC);
Revised: 15-Aug-2024, Manuscript No. IPHSJ-24-15061 (R);
Published:
22-Aug-2024, DOI: 10.36648/1791-809X.18.S11.003
Abstract
The World Health Organisation (WHO) and the United Nations International Children’s Emergency Fund (UNICEF) recommend zinc supplementation for children with diarrhea. However, Low and Middle-Income Countries (LMICs) conducted the majority of the studies supporting this recommendation. Although the mortality rate of acute diarrhea in developed countries is low, diarrhea leads to a high number of clinical care and hospital admissions, which represents a significant economic burden. This systematic review assessed the therapeutic benefits of zinc supplementation in the treatment of acute diarrhea in children living in high-income countries. We conducted a literature search on the Medline, Embase, Cochrane, and SciELO databases to find published randomised controlled trials on zinc supplementation and acute diarrhea in children residing in developed countries. We conducted a systematic literature search of the databases, uncovered 609 titles, and included 3 trials, totalling 620 treated children with acute diarrhea, after reviewing abstracts and full manuscripts for inclusion and exclusion criteria. Two studies showed that zinc did not interfere with the duration of diarrhea. According to the Cochrane Risk of Bias RoB 2, risk was considered low in two studies and some concerns in another. There was no statistically significant reduction in the mean RR for the occurrence of diarrheal episodes after 7 days of zinc supplement administration (0.4% vs. 0.6%; RR 0.73; 95% CI 0.28-1.92; p=0.53; I2=16%). Zinc supplementation did not reduce the duration of acute diarrhea among children living in developed countries.
Keywords
Acute diarrhea; Zinc supplementation; Children; High-income countries
Introduction
Diarrhea is the third leading cause of death in children 1–59
months of age, most of which occur in developing countries
[1]. According to the World Health Organization (WHO), acute
diarrhea is defined as the discharge of loose stools or liquid stools
≥ 3 times per day for ≥ 3 days and <14 days [2]. Diarrhea can quickly
lead to fluid and electrolyte loss and may be life-threatening,
especially in young infants and malnourished children. Besides,
diarrhea promotes nutritional deficiencies, reduces immunity,
and impairs growth and development [3-5].
Worldwide, zinc deficiency is common, but reports of severe
deficiency are rare [6]. Zinc is an important micronutrient for
cellular growth, cellular differentiation, and metabolism [7].
Zinc deficiency may limit immunity and impair resistance to
infections. Many studies have shown that zinc supplementation
may reduce the duration and severity of diarrhea [8-11]. A
systematic review of randomised controlled trials found that
oral zinc supplementation significantly reduces the duration
of diarrhea; however, only one of the 18 included studies took
place in a developed country [12]. Another systematic review
observed no consistent benefit with zinc trials to treat diarrhea
[13]. A Cochrane systematic review suggests that zinc may be of
benefit to children aged six months or more in areas where the
prevalence of zinc deficiency or malnutrition is high [14].
The World Health Organisation (WHO) and the United Nations
International Children’s Emergency Fund (UNICEF) recommend
zinc supplementation for children with acute diarrhea [15], last updated on 9 August, 2023. However, few studies were conducted
in developed countries, thereby limiting the global World
Health Organisation (WHO) recommendations for diarrhea. This
systematic review aims to assess the therapeutic benefits of zinc
supplementation in the treatment of acute diarrhea in children
living in high-income countries.
Materials and Methods
We developed this systematic review with meta-analysis in
accordance with the Cochrane handbook for systematic reviews
of interventions and reported it using the updated Preferred
Reporting Items for Systematic Reviews and Meta-Analyses
(PRISMA) Checklist [16]. We registered this study under Prospero
(CRD42024516946). Ethical approval is not required for this
study, as it is a systematic review.
We conducted a literature search using Medline, Embase,
Cochrane, and Scielo electronic databases, without any language
restrictions. We selected only Randomised Controlled Trials (RCTs)
conducted in developed countries. The search included trials that
were published in any language. Two reviewers (Túlio Revoredo
and João Guilherme Bezerra Alves) assessed the eligibility of
each record. Initially, we screened the title and abstract. At this
stage, we excluded studies that were not RCTs, not conducted
in developed countries, and did not include data on human
subjects, acute diarrhea, or oral zinc administration. We obtained
complete articles to conduct further reviews of pertinent studies.
A third reviewer (Lucas Victor Alves) resolved any disagreements
over the selection of studies.
The following keywords were utilized: "zinc", "zinc
supplementation", "oral zinc", "diarrhea", "acute diarrhea",
"diarrhea", and "randomised controlled trial", "randomised
clinical trial", or "randomised clinical trial". We used a form to
extract relevant data from studies, including year, country, study
design, population, setting, blinding, allocation concealment,
sample size, intervention, and outcomes. Moreover, to conduct
our statistical pooling, we solely extracted data from studies’
intention-to-treat analyses. Finally, we used the Web Plot
Digitizer tool to extract pertinent data from Kaplan-Meier curves
and incorporate it into our meta-analysis.
Eligibility criteria
According to the World Bank criteria, this review included RCTs
conducted in developed countries, while it excluded studies
conducted in Low and Middle-Income Countries (LMIC).
We selected studies that only included children with acute
diarrhea. The intervention consists of oral zinc administration
alone, without any combination. The primary outcome was the
duration of diarrhea. Secondary outcomes were stool frequency
(measured by the exact number of defecations recorded per day),
vomiting duration, hospitalisation and death from diarrhea.
Risk-of-bias assessment
The Risk-of-Bias RoB 2 Toll evaluated the risk of bias of included
RCTs based on seven domains:
• The randomization process;
• Deviations from intended intervention;
• Missing outcome data;
• Outcome measurement;
• Selection of the reported results;
• Incomplete reporting; and
• power calculation/sample size.
Before assessment, the reviewers were trained [17]. A third
reviewer (Lucas Victor Alves) resolved any disagreements.
Statistical analysis
We compared dichotomous endpoints using Risk Ratios (RR)
and 95% confidence intervals. P values ≥ 0.05 were considered
significant for the rejection of the null hypothesis that there were
no differences in effects between interventions. We adopted
the Mantel-Haenszel test for dichotomous data. We used a
DerSimonian and Laird random-effects model to incorporate the
assumption that true effect sizes varied between studies.
Moreover, to measure and analyse heterogeneity, we utilised the
Cochran Q test and I2 statistics. P values ≥ 0.10 were considered
significant for the rejection of the null hypothesis that the studies
shared a common true effect size. We used the I2 statistic to
assess the percentage of variance in observed effect sizes due
to heterogeneity. Due to the small number of included studies,
we opted not to incorporate prediction intervals to assess
heterogeneity or funnel plots to search for publication bias.
We used Cochrane's Review Manager Web for statistical analysis.
Results
Searching the following databases yielded a total of 629 studies:
PubMed (305), Embase (214), Cochrane (101), and SciELO
[9]. After excluding duplicate manuscripts (303) and studies
performed in LMIC countries (322), 4 studies showed potential
relevance for the full analysis. We then screened the full texts of
the remaining 4 articles for eligibility, excluding one because it
did not perform the intervention with only zinc. As a result, this
systematic review included three articles [18-20] (Figure 1).
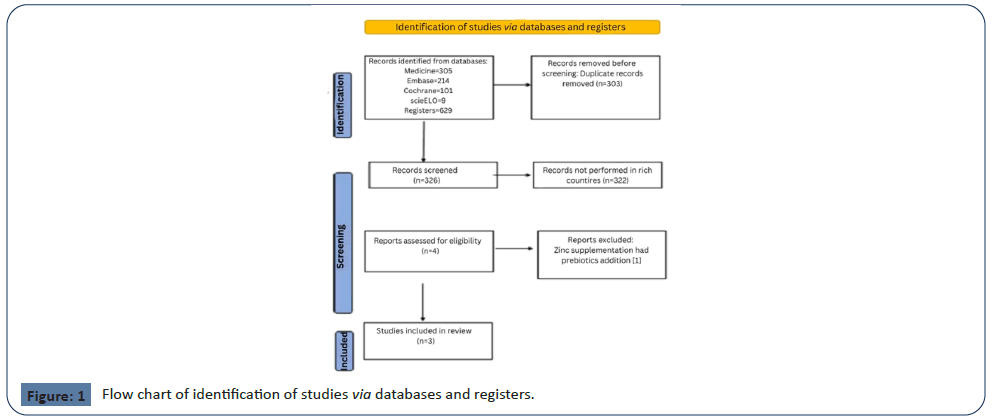
Figure 1: Flow chart of identification of studies via databases and registers.
The randomised controlled trial studies included 620 children
with acute diarrhea, with sample sizes ranging from 87 to 392.
The age of participants ranged from 3 months to <11 years old. Table 1 displays the characteristics of the included studies.
| Authors and year of publication |
Country |
Setting |
Design |
Allocation concealment |
Blinding |
Sample size |
Age (years) |
Duration of diarrhea (p) |
| Valery, et al. 2005 [18] |
Australia |
Hospital |
RCT |
Yes |
No |
392 |
<11 years |
0.69 |
| Patro, et al. (2010) [19] |
Poland |
Hospital |
RCT |
Yes |
Yes |
141 |
3 – 48 months |
>0.05 |
| Crisinel, et al. (2015) [20] |
Switzerland |
Hospital |
RCT |
Yes |
No |
87 |
3.1 - 50.3 months |
0.03 |
Table 1. Characteristics of studies included in this systematic review.
Valery et al., in Australia, studied 392 Aboriginal children, < 11
years old, with diarrhea supplemented with zinc, vitamin A, or
combined zinc and vitamin A. They found no significant effect
on the duration of diarrhea; the median diarrhea duration after
starting supplementation was 3.0 days for the zinc supplemented
and placebo groups (P values of 0.25 and 0.69, respectively).
Patro, et al., in Poland, studied 69 children in the zincsupplemented
group compared with 72 children in the control
group, and there was no significant difference in the duration of
diarrhea (P >.05). Similarly, they found no significant difference
in secondary outcome measures (frequent stool, vomiting,
intravenous fluid intake, and the number of children with diarrhea
lasting >7 days).
Crisinel et al., in Switzerland, studied 87 children (median age
14 months; range 3.1–58.3); 42 received zinc supplementation and 45 received placebo. There was no difference in the duration
or frequency of diarrhea, but only 5% of the zinc group still
had diarrhea at 120 hour of treatment, compared to 20% in
the placebo group (P = 0.05). The average length of diarrhea in
zinc-treated patients was 47.5 hours (18.3–72 hours), which was
significantly longer than the average length of diarrhea in the
placebo group (76.3 hours; IQR 52.8–137 hours) (P=0.03). The
frequency of diarrhea was also lower in the zinc group (P=0.02).
The primary outcome was determined by intention-to-treat
analysis, whereas the significant difference in median diarrhea
duration was determined by per-protocol analysis.
We have assessed the risk of bias in the included studies. Table 2 displays the risk of bias in the included studies. We classified two
studies as "low risk" [17,18], and one as "moderate risk" due to
missing data.
There was no statistically significant reduction in the mean
RR in the occurrence of diarrheal episodes after 7 days of zinc
supplement administration (0.4% vs. 0.6%; RR 0.73; 95% CI 0.28-
1.92; p = 0.53; I2 = 16%); The mean RR for comparable studies
could fall anywhere between 0.28 and 1.98, as represented by
the 95% confidence interval. In regards to heterogeneity, we still
can't reject the null hypothesis that all studies share a common
true effect size (p=0.30) through the use of Cochrane's Q statistic
(Figure 2, Table 3).
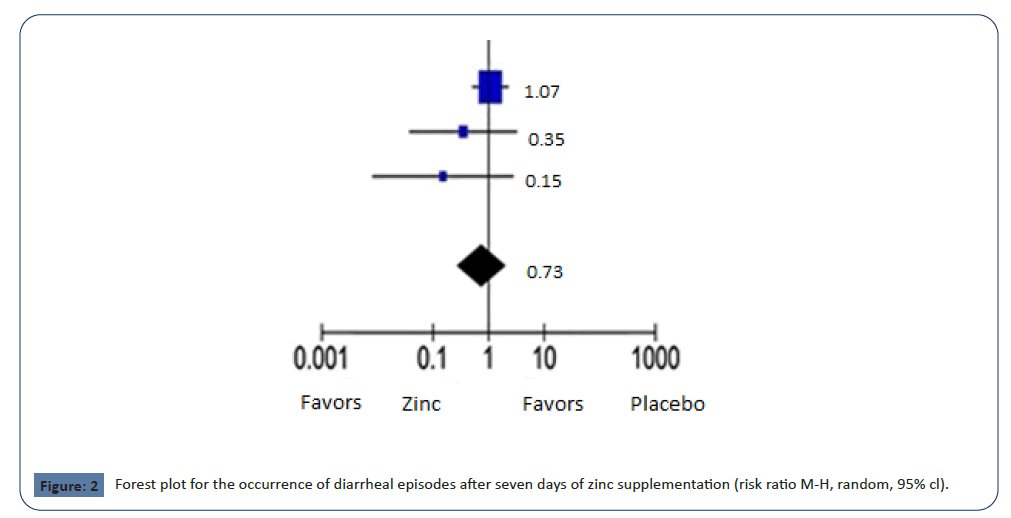
Figure 2: Forest plot for the occurrence of diarrheal episodes after seven days of zinc supplementation (risk ratio M-H, random, 95% cl).
| Study references |
Randomization |
Deviations from the intended intervention |
Missing outcome data |
Outcome Measurements |
Selective reporting |
Incomplete reporting |
Study power calculation/Sample size justification |
| Valery, et al. 2005 [18] |
(+) |
(+) |
(+) |
(+) |
(+) |
(+) |
(+) |
| Patro, et al. (2010) [19] |
(+) |
(+) |
(+) |
(+) |
(+) |
(+) |
(+) |
| Crisinel, et al. (2015) [20] |
(+) |
(+) |
(?) |
(+) |
(+) |
(+) |
(+) |
Table 2. Assessment of the risk of bias based on the Cochrane Risk of Bias 2 checklist. Note: (+) low risk of bias; (?) moderate risk of bias.
| |
Zinc |
Placebo |
Risk ratio |
| Study or subgroup |
Events |
Total |
Events |
Total |
Weight |
M-H, random, 95% cl |
| Valery, et al. 2005 [18] |
13 |
207 |
12 |
205 |
73.8% |
1.07 (0.50,2.30) |
| Patro, 2010 [19] |
1 |
69 |
3 |
72 |
16.3% |
0.35 (0.04,3.26) |
| Crisinel, 2015 [20] |
0 |
37 |
3 |
39 |
10.0% |
0.15 (0.01,2.82) |
| Total (95% cl) |
|
313 |
|
316 |
100.0% |
0.73 (0.28,1.92) |
| Total events |
14 |
|
18 |
|
|
|
Heterogeneity: Tau2=0.18; Chi2=2.38, df=2 (P=0.30); I2=16%
Test for overall effect: Z=0.63 (P=0.53)
Test for subgroup differences: Not applicable
Table 3. Forest plot for the occurrence of diarrheal episodes after 7 days of zinc supplementation.
Discussion
The findings of this systematic review did not suggest the
benefits of therapeutic zinc supplementation for diarrhea
among children in high-income countries, despite the detection
of only three randomised controlled trials. In rich countries,
the effects of zinc treatment, which include reductions in
episode duration, stool output, stool frequency, and length of
hospitalisation, were not the same as in studies performed in
low- and middle-income countries. These results suggest that
zinc therapy for diarrhea seems not to be beneficial in highincome
countries.
Patro, et al., observed no beneficial effect of zinc
supplementation on diarrhea duration or severity [18]. They
emphasise that they studied well-nourished and healthy
children, who were therefore unlikely to be zinc deficient. T The
authors attribute their inconsistent results with the majority of previously conducted trials, systematic reviews, and meta-analyses
that have demonstrated an anti-diarrheal effect of zinc in children
to the fact that these studies took place in countries with a medium
or low Human Development Index (HDI), where malnutrition and
zinc deficiency are significant issues. They also stated that countries
with a high or very high HDI have not conducted any studies
evaluating the effects of zinc for the treatment of acute diarrhea.
Valery, et al., came to the conclusion that hospitalised Aboriginal
children in Australia may not benefit from zinc supplementation in
the management of acute diarrhea [19]. However, they clarify that
their findings may not apply to children suffering from malnutrition.
They reported that their results for zinc supplementation differ from
those in other settings because supplementation is only effective
in populations with a low baseline level of these micronutrients.
They add that their results suggest that zinc supplementation has a
positive effect on stunted children.
This systematic review included only one study that found a
difference in the duration of diarrhea: 47.5 hours (18.3–72) in the
zinc group and 76.3 hours (52.8–137) in the placebo group (P =
0.03) [20]. However, this study has some limitations. First, they
were unable to recruit the expected number of patients based on
their calculations. Second, a large number of children were lost
to follow-up; 60 patients, out of a total of 148 patients recruited
for this study, were lost to follow-up without any available postbaseline
data. Third, they had poor compliance, probably due to
zinc's metallic taste.
Another study, which was not part of our systematic review,
showed that zinc supplementation had a positive effect on acute
diarrhea in a population of Italian children aged 3 to 36 months
(for a duration less than 24 hours) [21]. However, they used an
Oral Rehydration Solution (ORS) containing zinc and probiotics
in addition to oral zinc for the intervention. For this reason, the
question remains whether the effect was due to zinc or probiotics.
The possible zinc mechanisms of action against diarrhea are not
well understood. It may include improved absorption of water
and electrolytes by the intestine, better regeneration of the gut
epithelium, an increased number of enterocyte brush border
enzymes, and an enhanced immune response [22,23]. However,
it is questionable whether these actions are independent of zinc
deficiency in the host. This systematic review pointed to this.
Many randomised controlled trials performed in LMIC countries
have reported that oral zinc supplementation is effective in
reducing the duration of acute diarrhea, although some point to
divergent results [24-32]. Based on these results, the World Health
Organisation (WHO) and the United Nations Children’s Fund
(UNICEF) recommend oral zinc supplementation as a universal
treatment for all children with acute diarrhea (WHO/UNICEF)
[15]. On the other side, according to the recommendations of the
European Society for Paediatric Gastroenterology, Hepatology,
and Nutrition/European Society for Paediatric Infectious Diseases
(ESPGHAN/ESPID), there is not enough evidence to support its
routine use in children with acute diarrhea living in Europe,
where zinc deficiency is rare [33]. Although the mortality rate
of acute diarrhea in developed countries is low, diarrhea leads
to a high number of clinical care and hospital admissions, which
represents a significant economic burden.
Strengths and Limitations
The strength of this study is its pioneering use of synthesised
data from randomised clinical trials to evaluate the efficacy of
zinc supplementation for the management of acute diarrhea in
children living in developed countries. This study also followed all
the recommendations of the Cochrane Handbook for Systematic
Reviews of Interventions and reported according to the updated
Preferred Reporting Items for Systematic Reviews and Meta-
Analyses (PRISMA) checklist [16].
In regards to our meta-analysis, one limitation stems from
variations in the defined duration of diarrhea episodes. While Patro et al., provided data solely for the prevalence of episodes
lasting more than 7 days, [19] Valery et al., and Crisinel et al.,
reported data for episodes lasting 7 days or longer [18,20].
Despite this inconsistency, we chose to combine this data for our
pooling.
Additional limitations arise from variations in the age inclusion
criteria across studies. Patro et al., included children up to a
maximum of 4 years old (19), whereas Valery et al., extended
their inclusion criteria to children up to 11 years old. Thus, even if
we couldn’t reject the null hypothesis that heterogeneity wasn’t
present, this broader range likely does introduce heterogeneity
into our meta-analysis [18]. Furthermore, another potential
source of heterogeneity arises from the study by Valery et al., as
they included data on zinc supplementation among patients also
receiving vitamin A supplements, not providing stratified data for
patients receiving solely zinc supplementation against patients
receiving solely the placebo [18].
Finally, the primary constraint in our meta-analysis is undoubtedly
the limited number of studies included. In random-effects metaanalysis,
this poses a significant concern as it inhibits the accurate
calculation of between-study variance. Consequently, this may
lead to unreliable estimates for the summary effect, its associated
confidence interval, and the metrics pertaining to heterogeneity.
Hence, readers should exercise caution when interpreting our
results and consider their limited scope. Nevertheless, despite
these acknowledged limitations, conducting a meta-analysis is
preferable to relying on an ad-hoc summary of the evidence [34].
Conclusion
Zinc supplementation did not reduce the duration of acute
diarrhea among children living in developed countries. This result
supports the hypothesis that the anti-diarrheal effect of zinc is
dependent on zinc deficiency. The World Health Organisation
(WHO) and United Nations International Children's Emergency
Fund (UNICEF) recommended regimen of therapeutic zinc should
include only low and middle-income countries.
Highlights
New points in the study
• Zinc supplementation did not reduce the duration of acute
diarrhea among children living in developed countries.
• The anti-diarrheal effect of zinc is dependent on zinc
deficiency.
• The WHO and UNICEF recommended regimen of therapeutic
zinc should not include high-income countries.
Known points in the study
• Zinc supplementation may reduce the duration and severity
of diarrhea in poor countries.
• The World Health Organization (WHO) and the United
Nations International Children’s Emergency Fund (UNICEF)
recommend zinc supplementation for children with acute
diarrhea.
Declarations
Conflict of interest
The authors declare no conflict of interest
Funding
This study received no funding.
References
- Institute For Health Metrics and Evaluation (IHME) Global Burden of Diseases 2020.
- World Health Organization (WHO) Diarrhoeal disease 2024.
- Das JK, Bhutta ZA (2016) Global challenges in acute diarrhea Curr Opin Gastroenterol 32(1):18-23.
[Crossref] [Google Scholar] [PubMed]
- Fleckenstein JM, Matthew Kuhlmann F, Sheikh A (2021) Gastroenterol acute bacterial gastroenteritis Clin North Am 50(2):283-304.
[Crossref] [PubMed]
- Kisenge R, Dhingra U, Rees CA, Liu E, Dutta A, et al. (2024) Risk factors for moderate acute malnutrition among children with acute diarrhoea in India and Tanzania: A secondary analysis of data from a randomized trial BMC Pediatr 24:56.
[Crossref] [Google Scholar]
- Bailey RL, West KP Jr, Black RE (2015) The epidemiology of global micronutrient deficiencies Ann Nutr Metab 66(2):22-33.
[Crossref] [Google Scholar] [PubMed]
- Costa MI, Sarmento-Ribeiro AB, Gonçalves AC (2023) Zinc: From biological functions to therapeutic potential Int J Mol Sci 24(5):4822.
[Crossref] [Google Scholar] [PubMed]
- Gregorio GV, Dans LF, Cordero CP, Panelo CA (2007) Zinc supplementation reduced cost and duration of acute diarrhea in children J Clin Epidemiol 60(6):560-566.
[Crossref] [Google Scholar] [PubMed]
- Zou TT, Mou J, Zhan X (2015) Zinc supplementation in acute diarrhea Indian J Pediatr 82(5):415-420.
[Crossref] [Google Scholar]
- Lamberti LM, Walker CL, Chan KY, Jian WY, Black RE (2013) Oral zinc supplementation for the treatment of acute diarrhea in children: A systematic review and meta-analysis Nutrients 5(11):4715-4740.
[Crossref] [Google Scholar] [PubMed]
- Lazzerini M (2016) Oral zinc provision in acute diarrhea Curr Opin Clin Nutr Metab Care 19(3):239-243.
[Crossref] [Google Scholar] [PubMed]
- Galvao TF, Thees MFRS, Pontes RF, Silva MT, Pereira MG (2013) Zinc supplementation for treating diarrhea in children: A systematic review and meta-analysis. Rev Panam Salud Publica 33(5):370-377.
[Crossref] [Google Scholar] [PubMed]
- Pavlinac PB, Brander RL, Atlas HE, John-Stewart GC, Denno DM, et al. (2018) Interventions to reduce post-acute consequences of diarrheal disease in children: A systematic review BMC Public Health 18(1):208.
[Crossref] [Google Scholar] [PubMed]
- Lazzerini M, Wanzira H (2016) Oral zinc for treating diarrhoea in children Cochrane Database Syst Rev 12(12):CD005436.
[Crossref] [Google Scholar] [PubMed]
- WHO/UNICEF (2004) Clinical management of acute diarrhoea Geneva: WHO.
- Higgins JPT, Thomas J, Chandler J (2022) Cochrane handbook for systematic reviews of interventions Cochrane
[Google Scholar]
- Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, et al. (2019) RoB 2: A revised tool for assessing risk of bias in randomised trials BMJ 366:l4898.
[Crossref] [Google Scholar] [PubMed]
- Valery PC, Torzillo PJ, Boyce NC, White AV, Stewart PA, et al. (2005) Zinc and vitamin A supplementation in Australian indigenous children with acute diarrhoea: A randomised controlled trial Med J Aust 182(10):530-535.
[Crossref] [Google Scholar] [PubMed]
- Patro B, Szymański H, Szajewska H (2010) Oral zinc for the treatment of acute gastroenteritis in Polish children: A randomized, double-blind, placebo-controlled trial J Pediatr 157(6):984-988.e1.
[Crossref] [Google Scholar] [PubMed]
- Crisinel PA, Verga ME, Kouame KS, Pittet A, Rey-Bellet CG, et al. (2015) Demonstration of the effectiveness of zinc in diarrhoea of children living in Switzerland Eur J Pediatr 174(8):1061-1067.
[Crossref] [Google Scholar] [PubMed]
- Passariello A, Terrin G, De Marco G, Cecere G, Ruotolo S, et al. (2011) Efficacy of a new hypotonic oral rehydration solution containing zinc and prebiotics in the treatment of childhood acute diarrhea: A randomized controlled trial J Pediatr 158:288–292.e1.
[Crossref] [Google Scholar] [PubMed]
- Berni Canani R, Buccigrossi V, Passariello A (2011) Mechanisms of action of zinc in acute diarrhea Curr Opin Gastroenterol 27(1):8-12.
[Crossref] [Google Scholar] [PubMed]
- Hassan A, Sada KK, Ketheeswaran S, Dubey AK, Bhat MS (2020) Role of zinc in mucosal health and disease: A review of physiological, biochemical, and molecular processes 12(5):e8197.
[Crossref] [Google Scholar] [PubMed]
- Roy SK, Tomkins AM, Akramuzzaman SM, Behrens RH, Haider R, et al. (1997) Randomised controlled trial of zinc supplementation in malnourished Bangladeshi children with acute diarrhoea Arch Dis Child 77(3):196-200.
[Crossref] [Google Scholar] [PubMed]
- Strand TA, Chandyo RK, Bahl R, Sharma PR, Adhikari RK, et al. (2002) Effectiveness and efficacy of zinc for the treatment of acute diarrhea in young children Pediatrics 109(5):898-903.
[Crossref] [Google Scholar] [PubMed]
- Bhatnagar S, Bahl R, Sharma PK, Kumar GT, Saxena SK, et al. (2004) Zinc with oral rehydration therapy reduces stool output and duration of diarrhea in hospitalized children: A randomized controlled trial J Pediatr Gastroenterol Nutr 38(1):34-40.
[Crossref] [Google Scholar] [PubMed]
- Trivedi SS, Chudasama RK, Patel N (2009) Effect of zinc supplementation in children with acute diarrhea: Randomized double blind controlled trial Gastroenterology Res 2(3):168-174
[Crossref] [Google Scholar] [PubMed]
- Yazar AS, Güven Ş, Dinleyici EÇ (2016) Effects of zinc or synbiotic on the duration of diarrhea in children with acute infectious diarrhea Turk J Gastroenterol 27(6):537-540
[Crossref] [Google Scholar] [PubMed]
- Dhingra U, Kisenge R, Sudfeld CR, Dhingra P, Somji S, et al. (2020) Lower-dose zinc for childhood diarrhea - A randomized, multicenter trial N Engl J Med 383(13):1231-1241.
[Crossref] [Google Scholar] [PubMed]
- Rerksuppaphol L, Rerksuppaphol S (2020) Efficacy of zinc supplementation in the management of acute diarrhoea: A randomised controlled trial Paediatr Int Child Health 40(2):105-110.
[Crossref] [Google Scholar] [PubMed]
- Negi R, Dewan P, Shah D, Das S, Bhatnagar S, et al. (2015) Oral zinc supplements are ineffective for treating acute dehydrating diarrhoea in 5-12-year-olds Acta Paediatr 104(8):e367-371.
[Crossref] [Google Scholar] [PubMed]
- Wadhwa N, Natchu UC, Sommerfelt H, Strand TA, Kapoor V, et al. (2011) ORS containing zinc does not reduce duration or stool volume of acute diarrhea in hospitalized children J Pediatr Gastroenterol Nutr 53(2):161-167.
[Crossref] [Google Scholar] [PubMed]
- Guarino A, Albano F, Ashkenazi S, Gendrel D, Hoekstra JH, et al. (2008) The ESPGHAN/ESPID evidenced-based guidelines for the management of acute gastroenteritis in children in Europe J Pediatr Gastroenterol Nutr 46(2):S81-122.
[Crossref] [PubMed]
- Hedges LV, Higgins JPT, Rothstein HR, Borenstein M (2011) Introduction to Meta-Analysis John Wiley & Sons
[Crossref] [Google Scholar]
Citation: Revoredo T, Alves LV, Victor DR,
Alves JGB (2024) Zinc Supplementation in
the Management of Acute Diarrhea in High-
Income Countries – A Systematic Evaluation
and Meta-Analysis. Health Sci J Vol.18
No.S11:003







